Please cite this paper as:
Bitar, R.D., Torres-Garza, J.L., Reiter, R.J. and Phillips, W.T. 2021. Neural glymphatic system: Clinical implications and potential importance of melatonin. Melatonin Research. 4, 4 (Dec. 2021), 551-565. DOI:https://doi.org/https://doi.org/10.32794/mr112500111.
Review
Neural glymphatic system: Clinical implications and potential importance of melatonin
Ryan D. Bitar1, Jorge L. Torres-Garza,2 Russell J. Reiter,3 William T. Phillips4*
1Department of Radiology, Yale University School of Medicine, PO Box 208042 New Haven, CT 06520-8042
2University of Texas at San Antonio, One UTSA Cir, San Antonio, TX 78249-3209
3Cell Systems and Anatomy UT Health San Antonio 7703 Floyd Curl Drive San Antonio, Texas 78229-3800, USA
4Radiology, UT Health San Antonio 7703 Floyd Curl Drive, San Antonio, Texas 78229-3800, USA
*Correspondence: phillips@uthscsa.edu, Tel: +1-210 260-9128
Running title: The glymphatic system and melatonin
Received: September 6, 2021; Accepted: December 11, 2021
ABSTRACT
The central nervous system was thought to lack a lymphatic drainage until the recent discovery of the neural glymphatic system. This highly specialized waste disposal network includes classical lymphatic vessels in the dura that absorb fluid and metabolic by-products and debris from the underlying cerebrospinal fluid (CSF) in the subarachnoid space. The subarachnoid space is continuous with the Virchow-Robin peri-arterial and peri-vascular spaces which surround the arteries and veins that penetrate into the neural tissue, respectively. The dural lymphatic vessels exit the cranial vault via an anterior and a posterior route and eventually drain into the deep cervical lymph nodes. Aided by the presence of aquaporin 4 on the perivascular endfeet of astrocytes, nutrients and other molecules enter the brain from peri-arterial spaces and form interstitial fluid (ISF) that baths neurons and glia before being released into peri-venous spaces. Melatonin, a pineal-derived secretory product which is in much higher concentration in the CSF than in the blood, is believed to follow this route and to clear waste products such as amyloid-β from the interstitial space. The clearance of amyloid-β reportedly occurs especially during slow wave sleep which happens concurrently with highest CSF levels of melatonin. Experimentally, exogenously-administered melatonin defers amyloid-β buildup in the brain of animals and causes its accumulation in the cervical lymph nodes. Clinically, with increased age CSF melatonin levels decrease markedly, co-incident with neurodegeneration and dementia. Collectively, these findings suggest a potential association between the loss of melatonin, decreased glymphatic drainage and neurocognitive decline in the elderly.
Key words: neural glymphatic system, CSF, pineal gland, melatonin, neurodegeneration, dementia
___________________________________________________________________________________________________________
1. INTRODUCTION
The central nervous system (CNS), consisting primarily of the brain and spinal cord, serves a paramount role in the coordination and function of cognition and somatic processes (1). With the constant execution of multiple critical roles including but not limited to receiving sensory information, conducting motor commands, and processing cognitive activity, the brain has a high metabolic demand, accounting for 20% of the resting total oxygen consumption of the body (even though it accounts for only 2% of the total body weight) (1, 2). Additionally, neural tissue is extremely delicate, susceptible to severe damage from not only trauma but also from chemical substances, emphasizing the importance of the blood-brain barrier in the protection of the CNS from certain metabolites, drugs, and other toxic agents (1). Paradoxically, given its high metabolic demand and fragility, the CNS was thought to completely lack a lymphatic system for the clearance of metabolic waste products and other harmful solutes/substances until the recently-described glymphatic system (3).
The glymphatics are a macroscopic waste clearance system in the brain that utilizes perivascular channels, formed by astroglial cells, to promote efficient elimination of soluble proteins and metabolites from the central nervous system. The proper function of the glymphatic system is necessary for the CNS to perform optimally; however, given that the development of certain neurocognitive disorders can be characterized by the accumulation of macromolecules (alpha synuclein in Parkinson’s, beta amyloid and tau protein in Alzheimer’s, and superoxide dismutase in amyotrophic lateral sclerosis (ALS), the glymphatic system is believed to serve a neuroprotective role in neurocognitive disease via the clearance of macromolecules and solutes (4). Indeed, ensuring the proper long-term functionality of the glymphatic system may be a means of slowing or even halting the onset of neurocognitive disease. The discovery of factors which promote the protection and function of the glymphatic system offer promise for treatment of neurodegenerative processes. This article reviews the properties and physiology of the glymphatic system, modulators of its function, and proposes the potential importance of melatonin as a theoretical neuroprotective pharmaceutical agent to slow the progression of neurocognitive disease via action on and through the glymphatic system.
2. FLOW OF EXTRACELLULAR FLUID IN THE BRAIN
It is important to understand the general flux of extracellular fluid in the CNS in order to grasp the role of the glymphatic system. Cerebrospinal fluid (CSF) is a clear, proteinaceous fluid that bathes the CNS. With a formation rate of about 430-530 ml/day, there is about 150 ml of CSF circulating at any given moment (5). The majority (80%) of CSF is thought to be derived from the four choroid plexuses of the brain while the other 20% is hypothesized to come from metabolic water and leakage through the endothelial microcirculation (6). While the blood brain barrier is formed by endothelium containing tight junctions, fenestrated endothelium in the choroid plexus allow for passive diffusion of plasma into the choroidal interstitial space, followed by transport of molecules via ion channels and transporters across the choroid plexus epithelium and into the lumen of the ventricles (7), CSF then flows from the two lateral ventricles, to the third, and then the fourth ventricle and eventually through the foramina of Lushka and Magendie to fill the subarachnoid space. The classical pathway then notes that CSF is reabsorbed into outpouchings of the superior sagittal sinus via the subarachnoid granulations where CSF may reenter the bloodstream (8).
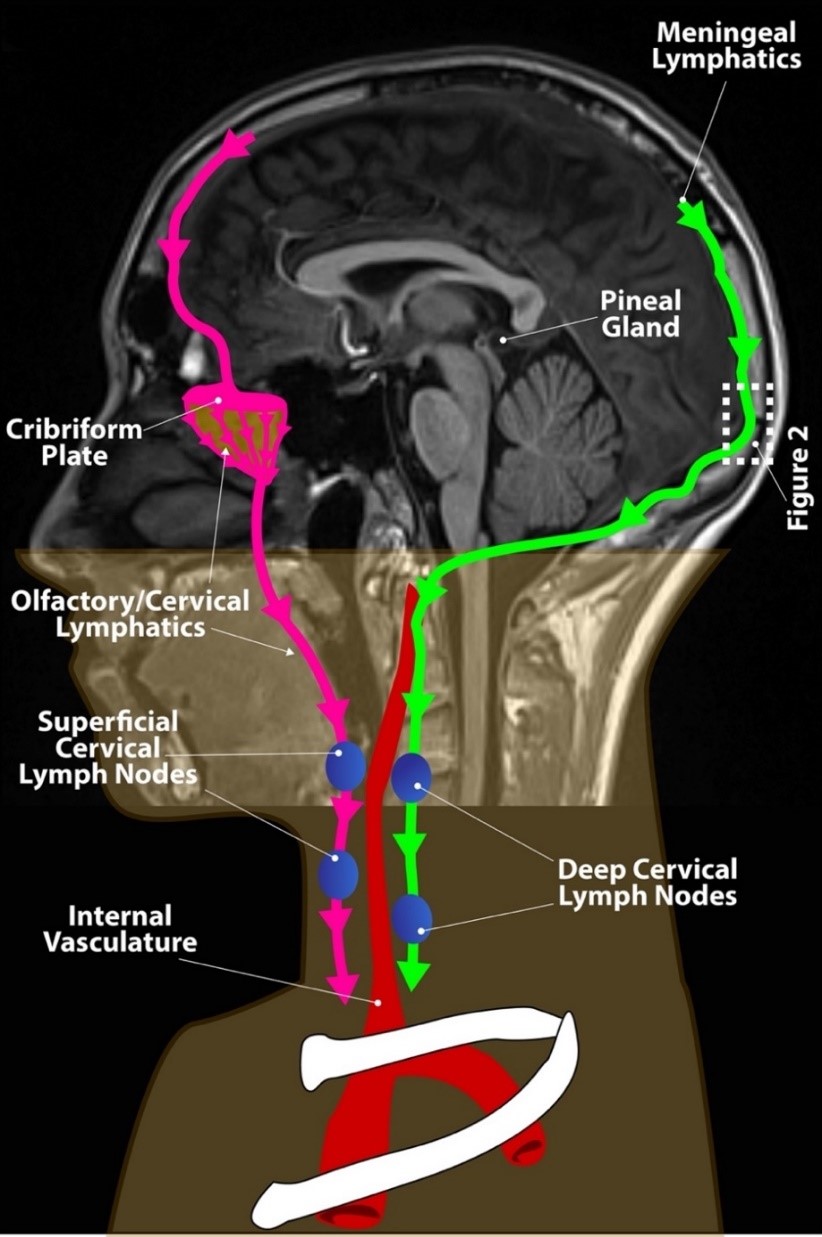
In recent years, alternative pathways for CSF efflux have been described, with many investigators considering clearance of CSF though perineural spaces of the cranial nerves, particularly the olfactory nerve to be the most dominant pathway of clearance of CSF with further transport to cervical lymphatics as shown in Figure 1 (9, 10).

Fig. 1. Brain lymphatic drainage.
In addition to the well known and previously described clearance of CSF by the arachnoid granulations extending from the arachnoid mater into the dural venous sinuses, alternative CSF clearance pathways are becoming recognized as important for clearance of the CSF from the brain including clearance of CSF when it accompanies the olfactory nerves through the cribriform plate to nasal lymphatics and the recently described posteriorly-positioned meningeal lymphatics. Both of these pathways eventually drain into lymphatic vessels that connect to cervical lymph nodes including the olfactory/cervical lymphatics shown in pink and the meningeal lymphatics shown in green. All of these pathways are fed by the glymphatic system. The pineal gland is shown which has secretion of melatonin into the third ventricle via the pineal recess resulting in high CSF concentrations and transport through the glymphatic system.
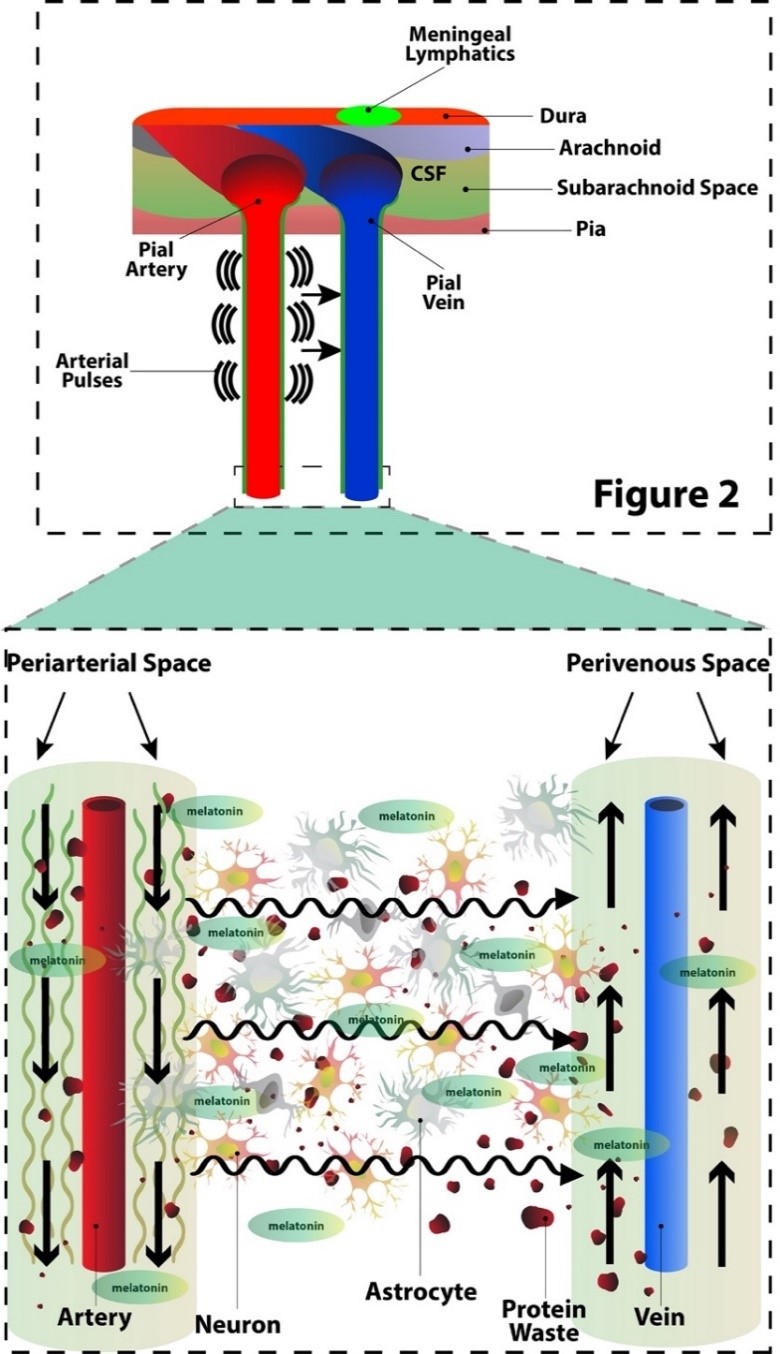
Fig. 2. The glymphatic system.
Magnification of the box from Figure 1. The glymphatic transit of CSF between the brain parenchymal cells is shown with incoming CSF flowing along the periarterial spaces of the pial arteries and moving toward the perivenous space of the pial veins. This glymphatic movement is thought to be assisted by convection from the arterial pulsations. Glymphatic movement is greatest during slow wave sleep when the interstitial space greatly expands and total movement of CSF increases up to 8-10 fold. This greatly increased movement of CSF during slow wave sleep occurs when melatonin secretion from the pineal gland is at its peak allowing melatonin to reach brain parenchymal cells. Melatonin is directly secreted from the pineal gland into the CSF as well as into the blood.
However, in regard to CSF movement within the subarachnoid space, a significant portion of the CSF may move directly into the brain parenchyma via the Virchow-Robin spaces (VRS), periarterial and perivenous spaces which surround all blood vessels that penetrate the brain parenchyma; this allows for the constant exchange of CSF with interstitial fluid of the brain (11). The VRS, filled with CSF, sheaths the penetrating pial arteries and veins as they dive into the parenchyma. The VRS then disappear prior to the capillary level so that the endothelium’s basal lamina directly touches the astrocyte endfeet. The periarterial CSF may then exchange with the ISF via the astrocyte endfeet which line the VRS which is then eventually released into the perivenous spaces for recirculation (3). This exchange of CSF with ISF with a net convective directionality of the fluid efflux from the periarterial spaces into the perivenous spaces forms the foundation of the glymphatic system as shown in Figure 2, first described in 2012 based on its glial water transport (12).
3. THE GLYMPHATIC SYSTEM
The glymphatic system plays a critical role in the clearance of waste metabolites and solutes from the brain parenchyma. The physiology and proper functionality of the glymphatic system is attributed to several factors which optimize the influx of CSF into the brain parenchyma. This flow happens at a remarkably expedited rate that cannot be attributable to simple diffusion alone. Indeed, a massive transport system is necessary to meet the demand of such a high flow of CSF (~500 ml per day) (13). Aquaporin-4 (AQP4) is an astroglial water channel abundantly expressed on the astrocytic endfeet which is thought to significantly regulate the exchange of CSF into the ISF. In 2018, Mestre and colleagues provided validation for this theory by demonstrating that an AQP-4 knockout rat model had a significantly decreased parenchymal CSF uptake and ISF efflux in comparison to the control rats (14). Experimental evidence also demonstrates that arterial pulsatility plays a significant role in the convective exchange of CSF and ISF. Iliff et al. in 2013 observed a significant reduction in fluid exchange after unilateral ligation of the internal carotid artery, whereas the rate of exchange was increased with infusion of dobutamine (15).
Wakefulness and sleep play instrumental roles in the functionality of the glymphatic system with sleep being very important for glymphatic function and its clearance of toxic metabolites and potentially aggregating proteins from the brain. A study conducted by Xie et al. (16) demonstrated that periarterial and parenchymal uptake of CSF tracer was reduced by 95% in awake mice as compared with sleeping mice. The cortical brain interstitial space was found to have increased by 60% during sleep, resulting in a more effective convective clearance of toxic metabolites from the parenchyma which is much more rapid than mere diffusion. The glymphatic activity is highest during the slow wave sleep which is associated with slow wave oscillations occurring every 30 seconds leading to metabolite clearance (16). This study, along with others indicate that the glymphatic system is most active during sleep and hindered during arousal (16). Additionally, posture during sleep may have a substantial effect on glymphatic function. Lee et al. conducted a study in rats demonstrating that CSF-ISF exchange was highest in rodents that slept in a lateral position rather than supine or prone (17).
4. MENINGEAL LYPHATICS
After discovery of the glymphatic system and further investigation of brain lymphatic-like clearance systems, a classical lymphatic vascular system was discovered in the dura mater of the meninges (Figure 1) (18, 19). Lymphatic vessels located in the dura mater absorb CSF from the adjacent subarachnoid space and brain interstitial fluid via the glymphatic system (20). Although these meningeal lymphatics are separated from the brain interstitial fluid by a tight arachnoid membrane, the glymphatic and meningeal lymphatic systems must be connected. Evidence for this connection is provided by studies of florescent tracers injected into the CSF of mice that document a rapid exit through the meningeal lymphatic vessels of the brain which then drain to deep cervical lymph nodes (21). Also, MRI imaging of rats have shown that contrast agents injected into the cisterna magna rapidly appear in the basal meningeal lymphatics and then moved to deep cervical nodes (21). Interestingly, MRI contrast was not found in the dorsal meningeal lymphatic vessels which have a zipper-like junctional pattern of lymphatic endothelial cells, whereas the capillary branches of the basal meningeal lymphatics have a different appearance with a discontinuously sealed loose button-like junctional pattern which is similar to traditional lymphatics in other parts of the body (21). In these studies, old mice were found to have decreased drainage of fluorescent quantum dots (~20 nm is diameter) from CSF to cervical lymph nodes, suggesting that aged basal meningeal vessels have decreased function as compared to younger lymphatic vessels (21). Certainly, glymphatic function during sleep and aging have important implications for neurodegenerative diseases of aging such as Alzheimer’s disease.
5. ALZHEIMER’S DISEASE
Alzheimers disease (AD) is a neurocognitive disorder which is characterized by the progressive decline in cognitive and functional capacity along with behavioral changes, which typically surface around the age of 65 (22). While age is the primary risk factor for the development of AD, other risk factors include the presence of the apolipoprotein gene E4 alleles, family history, traumatic brain injury, the female sex, sleep apnea, cardiovascular disease and metabolic syndrome (22, 23). AD is characterized by the abnormal accumulation of amyloid beta and tau in the brain. Amyloid beta are 36-43 amino acid peptides derived from amyloid precursor protein (APP) which results from the activities of beta secretase and gamma secretase. Misfolded amyloid beta peptides can accumulate and induce other amyloid beta molecules to misfold in the chair reaction that is similar to a prion infection. These misfolded and aggregated peptides form plaques which disrupt the healthy function of the brain (24). Unfortunately, to date, drugs that have been developed to treat AD by targeting secretase have not been successful (25). Aducanumab, is a monoclonal antibody that binds to amyloid administered for reduction of extracellular brain amyloid beta plaques. Although the therapeutic effect of aducanumab has been limited, the drug has received conditional approval for the treatment of this common neurodegenerative condition (26), although the recommendation was later narrowed to only patients with milder forms of AD.
Several pathological biomarkers have been used as research tools to diagnosis and monitor AD. These include 1) the ratio of 42 to 40 amino acid-long amyloid β which is a marker of plaque pathology; 2) total-tau and phosphorylated tau which are markers of AD-related changes in tau metabolism and secretion; and 3) neurofilament light as a marker of neurodegeneration (27). Given that the accumulation of amyloid and tau proteins pathologically characterizes AD and that the glymphatic pathway is a staple in the clearance of metabolic waste products and protein aggregates, it is reasonable to conjecture that the dysfunction of the glymphatic system would increase the risk of developing AD.
6. GLYMPHATIC SYSTEM AND ALZHEIMER’S DISEASE AND OTHER NEUROCOGNITIVE DISORDERS
The function of the glymphatic system is highly dependent on the proper polarization of AQP-4 water channels along the perivascular astrocyte foot processes and glial limiting membrane. AQP4 is a transmembrane tetramer which forms aqueous pores. It is not clear as to the biomolecular assortment of AQP4 along the astrocytic endfeet, however it has been suggested that higher order structures such as orthogonal arrays of particles (crystal-like supramolecular assemblies in the plasma membrane) may contribute to this preferential expression. It has been found that Dystrophin Associated Protein complex (DAPC) and its corresponding ligands (laminin and agrin) are necessary for AQP4 to cluster at the astrocyte endfeet (28). It has been demonstrated that glymphatic function is reduced in AD due to the loss of this quintessential polarization of the AQP4 protein (29).
Loss of polarization of AQP4 away from the astrocytic endfeet and into the parenchymal processes is a striking histological feature in mouse AD models and other diseases with reactive astrogliosis. It has been proposed that astrogliosis is another pathological hallmark of AD progression.(30) Indeed, CNS injury due to oxidative stress-induced DNA damage is a significant biomarker of neurodegenerative disease. Particularly, the 8-oxo-dG lesion is a common marker for patients with early symptoms of AD. Abnormally higher amounts of mitochondrial DNA oxidation products have been found in organisms with mild and advanced cognitive impairment (31). Amyloid aggregates or traumatic brain injury may form a reactive response from astroglia. Given that AQP4 facilitates astrocyte migration and glial scar formation, suggests the accumulation of beta amyloid and traumatic brain injury leads to the redistribution of AQP4 to the parenchymal endfeet of astrocytes, suggesting that loss of AQP4 is both a cause and consequence of dysfunction of the glymphatic system (32). A study conducted by Zeppenfeld et al (33), evaluated both the presence of amyloid beta and expression of AQP4 in the frontal cortex of postmortem human subjects. Expression of AQP4 was associated with advancing age in general; however, particularly the loss of perivascular localization of AQP4 was associated with a higher amyloid beta burden. Furthermore, symptoms of neurocognitive disorder were independent of age if the localization of AQP4 was preserved.
It is important to consider that AD is not a disease of old age; however, old age does induce a predisposition to the accumulation of amyloid beta aggregates, most likely attributable to the dysfunction of the glymphatic system. Kress et al (34), conducted a study to demonstrate and explain an age-related decline in the functionality of the glymphatic system in rodents. The study proved that in old mice, uptake of CSF tracer from the perivascular spaces across the pial surface was greatly reduced along with clearance of radiolabeled proteins. The study also discovered that AQP4 immunofluorescent signal was most prominent surrounding the arterioles in young mice; whereas in old mice, the AQP4 expression along the perivascular endfeet was somewhat reduced, but had increased along the tissue surrounding the penetrating arterioles, suggesting migration of AQP4. Additionally, age-related cerebrovascular disease may also explain the association of age with dysfunction of the glymphatic system.
It has been established that arterial pulsatility positively influences CSF-ISF exchange and that dampening of this pulsatility can reduce this flux. Cerebral arteries of aged patients exhibit reduced elastin expression and thus loss of distensibility and compliance. Particularly, amyloid angiopathy and atherosclerosis may be contributing factors towards the decreased pulsatility of cerebral perfusion related to aging (15, 35). Additionally, the association of old age with glymphatic dysfunction may be explained by age-related changes in sleep quality. Generally, the major age-related sleep changes are earlier wake times, reduced sleep consolidations, and poor sleep efficiency. The duration of slow-wave sleep experiences a remarkable decline in advanced age (36). Given that glymphatic clearance is highest during slow-wave sleep, elimination of toxic metabolites such as amyloid peptides may be suboptimal, possibly predisposing the elderly to AD. Evidence of the importance of sleep in normal subjects was shown by Kang et el. (37). They reported that in healthy young males, amyloid beta levels in the CSF increased during the day, peaked at night and decreased overnight. Given that quality sleep plays a critical role in neuroprotection from toxic metabolite and protein aggregates, any means of improving sleep quality may be important towards attenuating or potentially preventing AD. Many studies have demonstrated the melatonin improves sleep quality (38), therefore it is highly likely that this improved sleep quality due to melatonin use would be associated with more efficient clearance of metabolic proteins prone to aggregate in the brain.
The glymphatic system likely plays an important role in the development of other neurocognitive disorders in addition to Alzheimer’s disease (39). Nedergaard and Goldman hypothesized that glymphatic failure is a final common pathway to the development for illnesses such as Parkinson's Disease, Huntington's disease, multisystem atrophies, and frontotemporal dementia in addition to Alzheimer's disease. Given that the optimal functionality of the glymphatic system occurs during sleep and that these illnesses are strongly correlated with significant sleep disturbances, the authors indicate that there may be an association with the development of multiple types of dementia/neurocognitive disease with decline of the glymphatic system (40). Indeed, failure of the glymphatic system is thought to be the mechanism behind protein aggregation in the brain, which is considered to be the driving pathology behind several neurocognitive illnesses (24, 39).
7. MELATONIN
Melatonin, is a methoxindole produced and secreted by the pineal gland and traditionally found to be strongly associated with sleep-wake rhythms in humans (39). Pineal melatonin synthesis is upregulated during darkness and inhibited by light. Melatonin plays a critical role in regulation of the circadian rhythms along with its antioxidant, anti-inflammatory and anti-apoptotic actions which are manifested in all tissues including the CNS (40). The biosynthesis of melatonin involves four steps with the initial precursor being the essential amino acid, tryptophan. Synthesis begins with hydroxylation of tryptophan by tryptophan hydroxylase. The product, 5-hydroxytryptophan is then decarboxylated via 5-hydroxytryptophan decarboxylase to form serotonin. Serotonin is acetylated via N-acetyl transferase, the rate limiting enzyme in melatonin production, with the resultant generation of N-acetyl serotonin (41). Finally, N-acetyl serotonin is methylated by acetylserotonin methyltransferase to form melatonin. Pineal melatonin biosynthesis is highly regulated by a photoperiod-influenced suprachiasmatic nucleus (SCN), the master circadian clock. Additionally, melatonin has a feedback effect on the SCN to strengthen circadian rhythms. Loss of endogenous melatonin rhythm due to surgical removal of the pineal gland, light exposure at night or due to aging perturbs the circadian output of the SCN which interferes with the sleep:wake cycle (42).
In addition to the pineal gland, the mitochondria of neurons themselves synthesize melatonin. Neuronally-generated melatonin is never released into either the CSF or the blood; rather extracellular melatonin is produced by and secreted from the pineal gland. Suofu et al. demonstrated that neuronal mitochondria are capable of synthesizing melatonin from its precursor, serotonin, possess the key enzymes necessary for melatonin synthesis and that the methoxyindole functions as a potent antioxidant in the mitochondrial matrix (43). The synthesis of melatonin by neuronal mitochondria seems to be especially important given that these organelles are primary sites of reactive oxygen species (free radical) generation, especially in tissues such at the brain that utilize high levels of oxygen (44). The validation of the mitochondria as the cellular site of melatonin production supports the conjecture that melatonin functions as a physiological anti-inflammatory and anti-apoptotic agent since these processes are aggravated by free radicals Melatonin is known to reduce mitochondrial damage, to inhibit the release of cytochrome C and to attenuate caspase activation thereby reducing apoptosis. As with pineal melatonin synthesis, aging is believed to be a major factor in reducing melatonin production by mitochondria. Thus, elderly individuals are generally relatively deficient in both pineal-derived and neuronally-synthesized melatonin, which would be expected to hasten neural degeneration.
Melatonin plays a significant role in the regulation of cell autophagy in response to neurological stress such as brain injury or neurotoxicity. Interestingly, it functions both to promote a basal level of autophagy and to enhance healthy neuron survival but additionally, it limits an abnormally high degree of autophagy by suppressing the production of destructive oxygen and nitrogen-based reactants (45, 46). Given that neuroinflammation is a characteristic biological marker in AD patients, and the findings that melatonin reduced the levels of proinflammatory cytokines in the hippocampus of aged mouse brains, supports the conclusion that melatonin may be an effective neuroprotective agent (47, 48). Interestingly, melatonin’s anti-oxidative effects may even serve a critical role in pregnancy and fetal development. In the first trimester, human and rat placenta have significantly higher concentrations of melatonin than does serum (47). In the study by Wakatsuki et al, when melatonin was administered to pregnant rats, it protected against neural mitochondrial oxidative damage induced by hypoxanthine and xanthine oxidase, supporting the idea that melatonin plays an essential role in protecting the brain during fetal development (49).
While melatonin has well-documented antioxidant and anti-inflammatory actions, several of its metabolites also play key roles in protecting against neural degeneration resulting from trauma, stroke, toxic chemicals and drugs, and aging. These include N-acetylserotonin (NAS), a precursor in the melatonin synthesis pathway and the melatonin metabolites N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), cyclic 3-hydroxymelatonin (cOHM), 6-hydroxymelatonin (6OHM) and possibly others. Thus, some of the benefits ascribed to melatonin may derive from its metabolic kin. Melatonin protects against oxidative stress and neuronal mutilation by reducing the production of reactive oxygen species (ROS) via improving the efficiency of electron transfer through the electron transport chain; it also stimulates the activities of antioxidative enzymes, enhances the production of glutathione and by direct scavenging ROS. Less is known about the antioxidant capabilities of its precursor/metabolites, but they may be better direct radical scavengers than melatonin. Collectively, they provide formidable resistance to oxidative stress (50).
8. MELATONIN AND THE GLYMPHATIC SYSTEM
Melatonin induces sleep which is associated with a greatly increased clearance of toxic molecules from the glymphatic system. Maximum secretion of melatonin corresponds with the time when the glymphatic system is the most active suggesting that melatonin is integral to the process of waste removal and neuroprotection during sleep. Other evidence suggests that the glymphatic system is important for the movement of melatonin through the brain. Reiter et al have summarized the data showing that melatonin is released directly into the cerebrospinal fluid (CSF) of the third ventricle via the pineal recess (51). The direct release of melatonin into the CSF causes the levels of melatonin in this fluid to be at least an order of magnitude higher than concurrent levels in the blood. Melatonin then moves with the CSF throughout the ventricles and into the subarachnoid space as described above. From the subarachnoid space, melatonin follows the CSF in the periarterial spaces (VRS) where it has access to the brain parenchyma. The blood-brain barrier is no impediment to the entrance of melatonin into the interstitial space of the parenchyma; melatonin is known to easily cross this morphophysiological barrier.
The glymphatic system would appear to be an important transport system for moving high concentrations of melatonin released directly into the CSF from the pineal gland into neurons and glia throughout the CNS. The movement of high concentrations of melatonin through the glymphatic system has important implications for a variety of neurodegenerative diseases such as AD, Parkinson disease, Huntington disease, etc., all of which have a free radical and/or inflammatory component (51). With the aid of the senescence-associated mouse model, it has been documented that melatonin regulates amyloid beta production/clearance balance though effects on secretases as well as other actions (52).
The malfunction of the glymphatic system as it occurs with advancing age also coincides with the simultaneous reduction in melatonin production and secretion by the pineal gland. The age-associated drop in the amplitude of the nocturnal rise of melatonin seems to vary among individuals with the levels exhibiting the greatest reductions in the frail elderly relative to those in old people would are in relatively good health. This suggests there may be a relationship between melatonin and mental health in general with disturbances in the glymphatic sustem contributing to these debilitating conditions.
A number of authors have suggested that small molecules that may improve glymphatic function should be researched and developed (53, 54). Herein, we suggest that melatonin is a prime candidate as a small molecule that may have utility in the prevention and/or treatment of AD. This idea should be considered seriously since in experimental animals melatonin delays the progression of this devastating condition, because of its ability to readily cross the blood-brain barrier and its very high safety profile over a wide range of doses.
9. NEUROPROTECTIVE FUNCTIONS OF MELATONIN
Given the well-documented neuroprotective effects of melatonin, it is surmised that a deficiency of melatanin due to any reason, i.e., genetic, excessive nocturnal light exposure (e.g., night shift work), pineal pathology, advanced age, may heighten the risk/progression of neurodegenerative disease. The loss of melatonin has a number of consequences that could contribute to physiological/neurological decline with age. The loss of this key regulator leads to a perturbation of circadian rhythmicity, sleep cycles, a reduction in total antioxidant and anti-inflammatory activities all of which may further predispose the elderly to diminished neurological capacity (55). Additionally, melatonin has direct anti-amyloidogenic properties. Wang et al found that drug-mediated inhibition of melatonin synthesis in rats led to hyperphosphorylation of neurofilaments and cyclin dependent kinase 5 (CDK-5) activation leading to caspase activation and apoptosis. Reintroduction of exogenous melatonin partially arrested these molecular changes Similarly, Pappolla et al. have shown that melatonin inhibits beta-fibrillogenesis in AD (56, 57). Moreover, this group recently reported that treating animals with melatonin increased the leaching of beta-amyloid from the brain as indicated by the elevated levels of this protein in the cervical lymph nodes in a mouse model of amyloidosis (58). There is a vast literature related to the ability of melatonin to protect the brain from functional decline under a very wide range of conditions.
Considering the major importance of melatonin towards achieving and maintaining adequate sleep physiology and that the glymphatic system is most active during sleep, melatonin may serve an important role in the healthy function of the glymphatic system. Additionally, the demonstrated neuroprotective effects of melatonin in the CNS most likely maintain the proper physiologic function of the glymphatic system to prevent its functional decline. The potentially important implications behind the role of melatonin in the glymphatic system warrant future research dedicated to exploring how melatonin may be utilized for its protective effects on glymphatic system function.
10. CONCLUSION
It is evident that melatonin has great promise as an agent to delay/prevent the development of neurocognitive diseases given the ease with which it crosses the blood brain barrier, its promotion of proper sleep rhythms, its antioxidant and anti-inflammatory properties, its anti-amyloidogenic actions and its enhancement of the function of the glymphatic system. Melatonin regulates factors which promote the optimal function of the glymphatic system and enhance removal of protein aggregates and metabolic waste products which are correlated with the development of neurodegenerative disease. Melatonin directly regulates the circadian rhythm which optimizes quantity and quality of sleep over the same timeframe in which the glymphatic system is almost exclusively active. Additionally, melatonin provides neuroprotection by attenuating the effects of ROS, minimizing autophagy, apoptosis, and thus reactive gliosis. Given that reactive gliosis leads to scarring of the neural parenchyma and malregulated migration of the originally polarized AQP4 channels, melatonin may minimize the disturbance of the polarized net flow of extracellular fluid from the CSF into the interstitium and then the efflux into glymphatic perivenous spaces and meningeal lymphatic systems. In consideration of the findings summarized herein and a wealth of other data, it is reasonable to hypothesize that melatonin is a promising agent that may promote the preservation of proper functionality of the glymphatic system and potentially modulate the onset or progression of neurodegenerative disease. Considering the high safety of melatonin, future research should investigate the potential of a range of melatonin doses from moderate to high levels (10 mg to 100 mg) in older subjects with sleep disorders, in patients at increased risk of developing AD, patients with minimally impaired cognition (MIC) and patients with early AD. Additionally, investigation of forms of melatonin with prolonged and sustained release would also appear to be a worthy area of future investigations.
ACKNOWLEDGEMENT
The authors would like to thank Jonathan Sumner of UT Health San Antonio Creative Media Services for his help in drawing and formatting of the images in this article.
AUTHORSHIP
Ryan D. Bitar, MD participated in the concept/design, drafting and writing of the manuscript, and approval of the article. Jorge L. Torres-Garza, BS participated in the drafting and writing of the manuscript. Russel J. Reiter, PhD participated in the concept/design, drafting and writing of the manuscript, editing of the manuscript and approval of the article. William T. Phillips, MD participated in the concept/design, drafting and writing of the manuscript, editing of the manuscript and approval of the article.
CONFLICT OF INTEREST
None of the authors have a conflict of interest with the material in this article.
REFERENCES
Thau L, Reddy V, Singh P (2021) "Anatomy, Central Nervous System." in StatPearls (StatPearls Publishing, Treasure Island (FL).
Clarke DD, Sokoloff L (1999) Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th edition (Lippincott-Raven, Philadelphia.
Jessen NA,. Munk AS, Lundgaard I, Nedergaard M (2015) The glymphatic system: A Beginner's Guide. Neurochem. Res. 40: 2583-2599. http://dx.doi.org/10.1007/s11064-015-1581-6.
Nycz B, Mandera M (2021) The features of the glymphatic system. Auton. Neurosci. 232: 102774. http://dx.doi.org/10.1016/j.autneu.2021.102774.
Khasawneh AH, Garling RJ, Harris CA (2018) Cerebrospinal fluid circulation: What do we know and how do we know it? Brain. Circ. 4: 14-18. http://dx.doi.org/10.4103/bc.bc_3_18.
Benveniste H, Lee H, Volkow ND (2017) The glymphatic pathway: Waste removal from the CNS via cerebrospinal fluid transport. Neuroscientist 23: 454-465. http://dx.doi.org/10.1177/1073858417691030.
Damkier HH, Brown PD, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 93: 1847-1892. http://dx.doi.org/10.1152/physrev.00004.2013.
Proulx ST (2021) Cerebrospinal fluid outflow: a review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol. Life Sci. 78 (6): 2429-2457. doi: 10.1007/s00018-020-03706-5.
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11: 10-10. http://dx.doi.org/10.1186/2045-8118-11-10.
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, et al. (2004) Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 1: 2. http://dx.doi.org/10.1186/1743-8454-1-2.
Cherian I, Beltran M, Kasper EM, Bhattarai B, et al. (2016) Exploring the Virchow-Robin spaces function: A unified theory of brain diseases. Surg. Neurol. Int. 7: S711-S714. http://dx.doi.org/10.4103/2152-7806.192486.
Iliff JJ, Wang M, Liao Y, Plogg BA, et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4: 147ra111. http://dx.doi.org/10.1126/scitranslmed.3003748.
Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol. 17: 1016-1024. http://dx.doi.org/10.1016/s1474-4422(18)30318-1.
Mestre H, Hablitz LM, Xavier AL, Feng W et al. (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 7: e40070. http://dx.doi.org/10.7554/eLife.40070.
Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, et al. (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33: 18190-18199. http://dx.doi.org/10.1523/JNEUROSCI.1592-13.2013.
Xie L, Kang H, Xu Q, Chen MJ. et al. (2013) Sleep drives metabolite clearance from the adult brain. Science 342: 373-377. http://dx.doi.org/10.1126/science.1241224.
Lee H, Xie L, Yu M, Kang H, et al. (2015) The Effect of body posture on brain glymphatic transport. J. Neurosci. 35: 11034-11044. http://dx.doi.org/10.1523/JNEUROSCI.1625-15.2015.
Aspelund A, Antila S, Proulx ST, Karlsen TV, et al. (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212: 991-999. http://dx.doi.org/10.1084/jem.20142290.
Louveau A, Smirnov I,. Keyes TJ,. Eccles JD, et al. (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337-341. http://dx.doi.org/10.1038/nature14432.
Tamura R, Yoshida K, Toda M (2020) Current understanding of lymphatic vessels in the central nervous system. Neurosurg. Rev. 43: 1055-1064. http://dx.doi.org/10.1007/s10143-019-01133-0.
Ahn JH, Cho H, Kim JH, Kim SH, et al. (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572: 62-66. http://dx.doi.org/10.1038/s41586-019-1419-5.
Apostolova LG (2016) Alzheimer disease. Continuum (Minneap Minn) 22: 419-434. http://dx.doi.org/10.1212/CON.0000000000000307.
Ferini-Strambi L, Hensley M, Salsone M (2021) Decoding causal links between sleep apnea and Alzheimer's disease. J. Alzheimers Dis. 80: 29-40. http://dx.doi.org/10.3233/jad-201066.
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 8: 101-112. http://dx.doi.org/10.1038/nrm2101.
Miranda A, Montiel E, Ulrich H, Paz C (2021) Selective secretase targeting for Alzheimer's disease therapy. J. Alzheimers Dis. 81: 1-17. http://dx.doi.org/10.3233/jad-201027.
Patwardhan AG, Belemkar S (2021) An update on Alzheimer's disease: Immunotherapeutic agents, stem cell therapy and gene editing. Life Sci. 282: 1-9. 10.1016/j.lfs.2021.119790, 119790. http://dx.doi.org/10.1016/j.lfs.2021.119790.
Zetterberg H, Blennow K (2021) Moving fluid biomarkers for Alzheimer's disease from research tools to routine clinical diagnostics. Mol. Neurodegener. 16: 10. http://dx.doi.org/10.1186/s13024-021-00430-x.
Eidsvaag VA, Enger R, Hansson H-A, Eide PK, et al. (2017) Human and mouse cortical astrocytes differ in aquaporin-4 polarization toward microvessels. Glia. 65: 964-973. http://dx.doi.org/10.1002/glia.23138.
Mader S, Brimberg L (2019) Aquaporin-4 water channel in the brain and its implication for health and disease. Cells 8: 90. http://dx.doi.org/10.3390/cells8020090.
Verkhratsky A, V. Parpura, M. Pekna, M. Pekny et al. (2014) Glia in the pathogenesis of neurodegenerative diseases. Biochem. Soc. Trans. 42: 1291-1301. http://dx.doi.org/10.1042/bst20140107.
Hasan M, Genovese S, Fiorito S, Epifano F, et al. (2017) Oxyprenylated phenylpropanoids bind to MT1 melatonin receptors and inhibit breast cancer cell proliferation and migration. J. Nat. Prod. 80: 3324-3329. http://dx.doi.org/10.1021/acs.jnatprod.7b00853.
Smith AJ, Duan T, Verkman AS (2019) Aquaporin-4 reduces neuropathology in a mouse model of Alzheimer’s disease by remodeling peri-plaque astrocyte structure. Acta Neuropathol. Commun. 7: 74. http://dx.doi.org/10.1186/s40478-019-0728-0.
Zeppenfeld DM, Simon M. Haswell JD, D'Abreo D, et al. (2017) Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 74: 91-99. http://dx.doi.org/10.1001/jamaneurol.2016.4370.
Kress BT, Iliff JJ, Xia M, Wang M, et al. (2014) Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76: 845-861. http://dx.doi.org/10.1002/ana.24271.
Beach TG, Wilson JR, Sue LI, Newell A, et al. (2007) Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 113: 13-21. http://dx.doi.org/10.1007/s00401-006-0136-y.
Colten HR, Altevogt BM (2006) Sleep disorders and sleep deprivation: An unmet public health problem. Sleep Physiology (National Academies Press (US), Institute of Medicine (US) Committee on Sleep Medicine and Research, Washington (DC), 2006).
Kang JE, Lim MM, Bateman RJ, Lee JJ, et al. (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326: 1005-1007. http://dx.doi.org/10.1126/science.1180962.
Fatemeh G, Sajjad M, Niloufar R, Neda S, et al. (2021) Effect of melatonin supplementation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. J. Neurol. 10.1007/s00415-020-10381-whttp://dx.doi.org/10.1007/s00415-020-10381-w.
Claustrat B, Brun J, Chazot G (2005) The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 9: 11-24. http://dx.doi.org/10.1016/j.smrv.2004.08.001 (E-Pub Feb).
Chen D, Zhang T, Lee TH (2020) Cellular mechanisms of melatonin: Insight from neurodegenerative diseases. Biomolecules 10: 1158.
Reiter RJ (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 12: 151-180. http://dx.doi.org/10.1210/edrv-12-2-151.
Agez L, Laurent V, Guerrero HY, Pévet P, et al. (2009) Endogenous melatonin provides an effective circadian message to both the suprachiasmatic nuclei and the pars tuberalis of the rat. J. Pineal Res. 46: 95-105. http://dx.doi.org/10.1111/j.1600-079X.2008.00636.x.
Suofu Y, Li W, Jean-Alphonse FG, Jia J, et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U S A 114: E7997-E8006. http://dx.doi.org/10.1073/pnas.1705768114.
Reiter RJ, Ma Q, Sharma R (2020) Melatonin in mitochondria: mitigating clear and present dangers. Physiology (Bethesda) 35: 86-95. http://dx.doi.org/10.1152/physiol.00034.2019.
Su LY, Li H, Lv L, Feng YM, et al. (2015) Melatonin attenuates MPTP-induced neurotoxicity via preventing CDK5-mediated autophagy and SNCA/alpha-synuclein aggregation. Autophagy 11: 1745-1759. http://dx.doi.org/10.1080/15548627.2015.1082020.
Wongprayoon P, Govitrapong P (2017) Melatonin as a mitochondrial protector in neurodegenerative diseases. Cell Mol. Life Sci. 74: 3999-4014. http://dx.doi.org/10.1007/s00018-017-2614-x.
Hossain MF, Wang N, Chen R, Li S, et al. (2021) Exploring the multifunctional role of melatonin in regulating autophagy and sleep to mitigate Alzheimer’s disease neuropathology. Ageing Res. Rev. 67: 101304. http://dx.doi.org/https://doi.org/10.1016/j.arr.2021.101304.
Permpoonputtana K, Tangweerasing P, Mukda S, Boontem P, et al. (2018) Long-term administration of melatonin attenuates neuroinflammation in the aged mouse brain. EXCLI J. 17: 634-646. http://dx.doi.org/10.17179/excli2017-654.
Wakatsuki A, Okatani Y, Shinohara K, Ikenoue N, et al. (2001) Melatonin protects fetal rat brain against oxidative mitochondrial damage. J. Pineal Res. 30: 22-28. http://dx.doi.org/10.1034/j.1600-079x.2001.300103.x.
Alvarez-Diduk R, Galano A, Tan DX, Reiter RJ (2015) N-Acetylserotonin and 6-hydroxymelatonin against oxidative stress: Implications for the overall protection exerted by melatonin. J. Phys. Chem. B 119: 8535-8543. http://dx.doi.org/10.1021/acs.jpcb.5b04920.
Reiter RJ, Tan DX, Kim SJ, Cruz MH (2014) Delivery of pineal melatonin to the brain and SCN: role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 219: 1873-1887. http://dx.doi.org/10.1007/s00429-014-0719-7.
Li Y, Zhang J, Wan J, Liu A, et al. (2020) Melatonin regulates Abeta production/clearance balance and A beta neurotoxicity: A potential therapeutic molecule for Alzheimer's disease. Biomed. Pharmacother. 132: 110887. http://dx.doi.org/10.1016/j.biopha.2020.110887.
Nedergaard M, Goldman SA (2020) Glymphatic failure as a final common pathway to dementia. Science 370: 50-56. http://dx.doi.org/10.1126/science.abb8739.
Sun BL, Wang LH, Yang T, Sun JY, et al. (2018) Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog. Neurobiol. 163-164: 118-143. http://dx.doi.org/10.1016/j.pneurobio.2017.08.007.
Shukla M, Govitrapong P, Boontem P, Reiter RJ, et al. (2017) Mechanisms of melatonin in alleviating Alzheimer's disease. Curr. Neuropharmacol. 15: 1010-1031. http://dx.doi.org/10.2174/1570159X15666170313123454.
Wang S, Zhu L, Shi H, Zheng H., t al. (2007) Inhibition of melatonin biosynthesis induces neurofilament hyperphosphorylation with activation of cyclin-dependent kinase 5. Neurochem. Res. 32: 1329-1335. http://dx.doi.org/10.1007/s11064-007-9308-y.
Pappolla MA, Bozner P, Soto C, Shao H, et al. (1998) Inhibition of Alzheimer β-fibrillogenesis by melatonin. J. Biol. Chem. 273: 7185-7188. http://dx.doi.org/https://doi.org/10.1074/jbc.273.13.7185.
Pappolla MA, Matsubara E. Vidal R, Pacheco-Quinto J, et al. (2018) Melatonin treatment enhances Aβ lymphatic clearance in a transgenic mouse model of amyloidosis. Curr. Alzheimer Res. 15: 637-642. http://dx.doi.org/10.2174/1567205015666180411092551.

This work is licensed under a Creative Commons Attribution 4.0 International License