Functional characterization of tobacco (Nicotiana benthamiana) serotonin N-acetyltransferases (NbSNAT1 and NbSNAT2)
Functional analysis of tobacco SNAT genes
Abstract
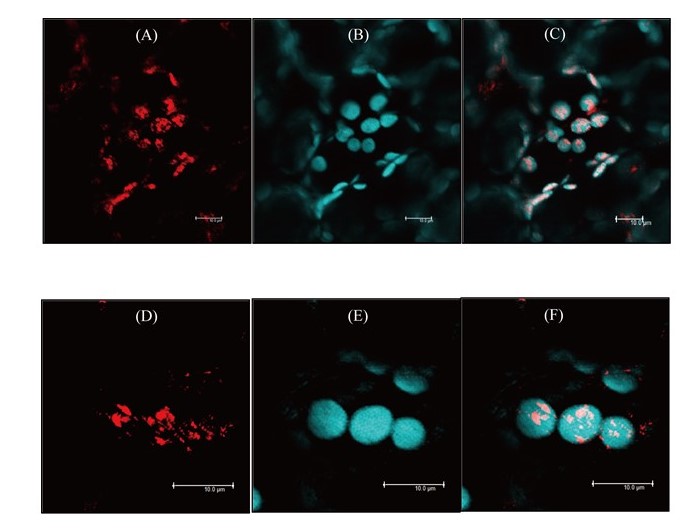
Nicotiana benthamiana (tobacco) is an important dicotyledonous model plant; however, no serotonin N-acetyltransferases (SNATs) have been characterized in tobacco. In this study, we identified, cloned, and characterized the enzyme kinetics of two SNAT genes from N. benthamiana, NbSNAT1 and NbSNAT2. The substrate affinity (Km) and maximum reaction rate (Vmax) for NbSNAT1 were 579 µM and 136 pkat/mg protein for serotonin, and 945 µM and 298 pkat/mg protein for 5-methoxytryptamine, respectively. Similarly, the Km and Vmax values for NbSNAT2 were 326 µM and 26 pkat/mg protein for serotonin, and 872 µM and 92 pkat/mg protein for 5-methoxytryptamine, respectively. Moreover, we found that NbSNAT1 and NbSNAT2 localized to chloroplasts, similar to SNAT proteins from other plant species. The activities of the NbSNAT proteins were not affected by melatonin feedback inhibition in vitro. Finally, transgenic tobacco plants overexpressing either NbSNAT1 or NbSNAT2 did not exhibit increased melatonin levels, possibly due to the expression of catabolic enzymes. Generating transgenic tobacco plants with downregulated NbSNAT expression would provide further insight into the functional role of melatonin in tobacco plants.
References
2. Arnao MB, Hernández-Ruiz J (2021) Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 23: 7-19.
3. Wang Y, Reiter RJ, Chan Z (2018) Phytomelatonin: a universal abiotic stress regulator. J. Exp. Bot. 69: 963-974.
4. Arnao MB, Hernández-Ruiz J (2020) Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 33: 77-87.
5. Back K (2021) Melatonin metabolism, signaling and possible roles in plants. Plant J. 105: 376-391.
6. Lee HY, Back K (2018) Melatonin plays a pivotal role in conferring tolerance against endoplasmic reticulum stress via mitogen-activated protein kinases and bZIP60 in Arabidopsis thaliana. Melatonin Res. 1: 93-107.
7. Lee HY, Back K (2021) Melatonin regulates chloroplast protein quality control via a mitogen-activated protein kinase signaling pathway. Antioxidants 10: 511.
8. Hong Y, Zhang Y, Sinumporn S, Yu N, Zhan X, Shen X, Chen D, Yu P, Wu W, Liu Q, Cao Z, Zhao C, Cheng S, Cao L (2018) Premature leaf senescence 3, encoding a methyltransferase, is required for melatonin biosynthesis in rice. Plant J. 95: 877-891.
9. Lu H, Luo T, Fu H, Wang L, Tan Y, Huang J, Wang Q, Ye G, Gatehouse AMR, Lou Y, Shu Q (2018) Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 4: 338-344.
10. Zheng Y, Xu J, Wang F, Tang Y, Wei Z, Ji Z, Wang C, Zhao K (2021) Mutation types of CYP71P1 cause different phenotypes of mosaic spot lesion and premature leaf senescence in rice. Front. Plant Sci. 12: 641300.
11. Dyda F, Klein DC, Hickman AB (2000) GCN5-related N-acetyltransferases: A structural overview. Annu. Rev. Biophys. Biomol. Struct. 29: 81-103.
12. Kang K, Lee K, Park S, Byeon Y, Back K (2013) Molecular cloning of rice serotonin N-acetyltransferase, the penultimate gene in plant melatonin biosynthesis. J. Pineal Res. 55: 7-13.
13. Byeon Y, Lee HY, Back K (2016) Cloning and characterization of the serotonin N-acetyltransferase-2 gene (SNAT2) in rice (Oryza sativa). J. Pineal Res. 61: 198-207.
14. Lee HY, Byeon Y, Lee K, Lee HJ, Back K (2014) Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localization. J. Pineal Res. 57: 418-426.
15. Lee HY, Lee K, Back K (2019) Knockout of Arabidopsis serotonin N-acetyltransferase-2 reduces melatonin levels and delays flowering. Biomolecules 9: 712.
16. Li C, He Q, Zhang F, Yu J, Li C, Zhao T, Zhang Y, Xie Q, Su B, Mei L, Zhu S, Chen J (2019) Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J. 100: 784-800.
17. Yu Y, Bian L, Jiao Z, Keke Y, Wan Y, Zhang G, Guo D (2019) Molecular cloning and characterization of a grapevine (Vitis vinifera L.) serotonin N-acetyltransferase (VvSNAT2) gene involved in plant defense. BMC Genomics 20: 880.
18. Wang X, Zhang H, Xie Q, Liu Y, Lv H, Bai R, Ma R, Li X, Zhang X, Guo YD, Zhang N (2020) SISNAT interacts with HSP40, a molecular chaperone, to regulate melatonin biosynthesis and promote thermotolerance in tomato. Plant Cell Physiol. 61: 909-921.
19. Bombarely A, Rosli HG, Vrebalov J, Moffett P, Mueller LA, Martin GB (2012) A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Mol. Plant-Microbe Interact. 25: 1523-1530.
20. Byeon Y, Lee HY, Lee K, Park S, Back K (2014) Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT. J. Pineal Res. 56: 107-114.
21. Karimi M, Inze D, Depocker A (2002) Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7: 193-195.
22. Duan W, Wang L, Song G (2016) Agrobacterium tumefaciens-mediated transformation of wild tobacco species Nicotiana debneyi, Nicotiana clevelandii, and Nicotiana glutinosa. Am. J. Plant Sci. 7: 1-7.
23. Byeon Y, Back K (2016) Melatonin production in Escherichia coli by dual expression of serotonin N-acetyltransferase and caffeic acid O-methyltransferase. Appl. Microbiol. Biotechnol. 100: 6683-6691.
24. Lee, HY, Back K (2020) The phytomelatonin receptor (PMRT1) Arabidopsis Cand2 is not a bona fide G protein-coupled melatonin receptor. Melatonin Res. 3: 177-186.
25. Emanuelsson O, Nielsen H, Brunak S, Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300: 1005-1016.
26. Park S, Byeon Y, Lee HY, Kim YS, Ahn T, Back K (2014) Cloning and characterization of a serotonin N-acetyltransferase from a gymnosperm, loblolly pine (Pinus taeda). J. Pineal Res. 57: 348-355.
27. Park S, Back K (2012) Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J. Pineal Res. 53: 385-389.
28. Kobylińska A, Reiter RJ, Posmyk MM (2017) Melatonin protects cultured tobacco cells against lead-induced cell death via inhibition of cytochrome C translocation. Front. Plant Sci. 8: 1560.
29. Domingos ALG, Hermsdorff HHM, Bressan J (2019) Melatonin intake and potential chronobiological effects on human health. Crit. Rev. Food Sci. Nutr. 59: 133-140.
30. Xu T, Chen Y, Kang H (2019) Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front. Plant Sci. 10: 1388.
31. Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 61: 426-437.
32. Liao L, Zhou Y, Xu Y, Zhang Y, Liu X, Liu B, Chen X, Guo Y, Zeng Z, Zhao Y (2021) Structural and molecular dynamics analysis of plant serotonin N-acetyltransferase reveal an acid/base-assisted catalysis in melatonin biosynthesis. Angew. Chem Int. Ed. 60: 12020-12026.
33. Zheng S, Zhu Y, Liu C, Fan W, Xiang Z, Zhao A (2021) Genome-wide identification and characterization of genes involved in melatonin biosynthesis in Morus notabilis (wild mulberry). Phytochemistry 189: 112819.
34. Wang L, Feng C, Zheng X, Guo Y, Zhou F, Shan D, Liu X, Kong J (2017) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 63: e12429.
35. Byeon Y, Lee HY, Choi DW, Back K (2015) Chloroplast encoded serotonin N-acetyltransferase in the red alga Pyropia yezoensis: gene transition to the nucleus from chloroplasts. J. Exp. Bot. 66: 709-717.
36. Tan DX, Hardeland R, Back K, Manchester LC, Alatorre-Jimenez MA, Reiter RJ (2016) On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: comparisons across species. J. Pineal Res. 61: 27-40.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


