Oral administration of melatonin increases plasma calcium and magnesium and improves bone metabolism in aged male mice
Melatonin regulated plasma divalent ions levels
Abstract
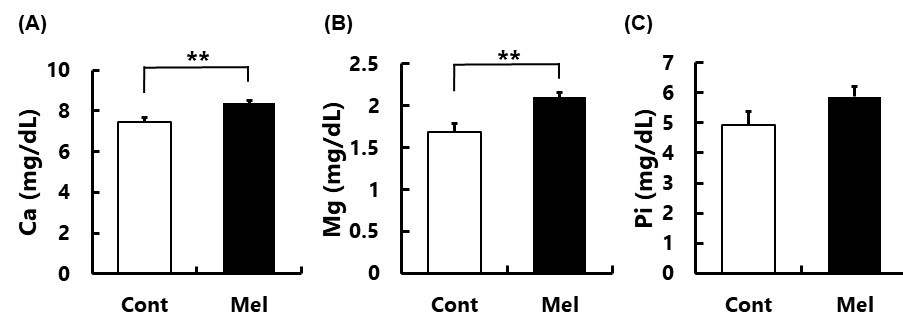
We previously reported that the oral administration of melatonin from 4 to 20 months to male mice improved femoral bone strength and bone density during the aging. Additionally, melatonin receptor, MT2, was immunologically detected in both osteoblasts and osteoclasts of the mouse femoral bone. Thus, melatonin can act on both osteoblasts and osteoclasts to maintain bone strength during the aging process. Here, we analyzed plasma calcium (Ca2+), magnesium (Mg2+), and inorganic phosphorus ([PO4]3-) in 20-month-old male mice with or without administration melatonin (15-20 mg/kg/day) in drinking water. We found that plasma Ca2+ and Mg2+ levels in melatonin-treated mice increased significantly as compared with control mice. In [PO4]3-, melatonin administration tended to increase its plasma level, but did not reach statistical significance. The potential association between these divalent ions and metabolism markers of femoral bone was also examined. In the femoral diaphysis, the plasma Ca2+ and Mg2+ concentrations were positively correlated with periosteal and endosteal circumference which were significantly associated with the Strength Strain Index. Therefore, melatonin treatment enlarged femoral diaphysis and enhanced bone strength by increasing mineral depositions. In addition, the plasma melatonin levels were significantly positive correlation with total bone density and critical thickness in the femoral diaphysis. Since we had not observed the primary trabecular bone and osteoclasts in 20-month-old mice previously, it is suggested that plasma Ca2+ and Mg2+ are not elevated due to bone resorption. The increased plasma Ca2+ and Mg2+ by melatonin may originate from the intestinal absorption of these ions since melatonin binds to the vitamin D3 receptor, its activation is known to promote the intestinal absorption of Ca2+.
References
2. Lewy AJ, Emens J, Jackman A, Yuhas K (2006) Circadian uses of melatonin in humans. Chronobiol. Int. 23: 403-412. https://doi.org/10.1080/07420520500545862.
3. Wei S, et al. (2020) Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: A meta-analysis of randomized controlled trials. Sleep. Med. 68: 1-8. https://doi.org/10.1016/j.sleep.2019.02.017.
4. Suzuki N, Hattori A (2002) Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J. Pineal. Res. 33: 253-258. https://doi.org/10.1034/j.1600-079X.2002.02953.x.
5. Koyama H, Nakade O, Takada Y, Kaku T, Lau KHW (2002) Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J. Bone Miner. Res. 17: 1219–1229. https://doi.org/10.1359/jbmr.2002.17.7.1219.
6. Suzuki N, Somei M, Seki A, Reiter RJ, Hattori A (2008) Novel bromomelatonin derivatives as potentially effective drugs to treat bone diseases. J. Pineal Res. 45: 229-234. https://doi.org/10.1111/j.1600-079X.2008.00623.x.
7. Maria S, et al. (2017) Melatonin-micronutrients Osteopenia Treatment Study (MOTS): A translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging 9: 256-285. https://doi.org/10.18632/aging.101158.
8. Maria S, et al. (2018) Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 64: e12465. https://doi.org/10.1111/jpi.12465.
9. Ikegame M, et al. (2019) Melatonin is a potential drug for the prevention of bone loss during space flight. J. Pineal Res. 67: e12594. https://doi.org/10.1111/jpi.12594.
10. Wang X, et al. (2019) Melatonin prevents bone destruction in mice with retinoic acid–induced osteoporosis. Mol. Med. 25: 43. https://doi.org/10.1186/s10020-019-0107-0.
11. Bell NH, Johnson RH (1997) Bisphosphonates in the treatment of osteoporosis. Endocrine 6: 203-206. https://doi.org/10.1007/BF02738966.
12. Davis S, et al. (2016) A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health. Technol. Assess. 20: 1-406. https://doi.org/10.3310/hta20780.
13. McClung M, et al. (2013). Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am. J. Med. 126: 13-20. https://doi.org/10.1016/j.amjmed.2012.06.023.
14. Reyes C, Hitz M, Prieto-Alhambra D, Abrahamsen B (2016) Risks and benefits of bisphosphonate therapies. J. Cell Biochem. 117: 20-28. https://doi.org/10.1002/jcb.25266.
15. Igarashi-Migitaka J, et al. (2020) Oral administration of melatonin contained in drinking water increased bone strength in naturally aged mice. Acta Histochem. 122: 151596. https://doi.org/10.1016/j.acthis.2020.151596.
16. Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T (2010) Genetic variation of melatonin productivity in laboratory mice under domestication. Proc. Natl. Acad. Sci. USA 107: 6412-6417. https://doi.org/10.1073/pnas.0914399107.
17. Kennaway DJ (2019) Melatonin-deficient Balb/c mice and their use in cancer research. Cancer Control 26: 1-2. https://doi.org/10.1177/1073274819886825.
18. Zoroddu MA, et al. (2019) The essential metals for humans: A brief overview. J. Inorg. Biochem. 195: 120-129. https://doi.org/10.1016/j.jinorgbio.2019.03.013.
19. Bonjour JP (2011) Calcium and phosphate: A duet of ions playing for bone health. J. Am. Coll. Nutr. 30: 438S-448S. https://doi.org/10.1080/07315724.2011.10719988.
20. Murshed M (2018) Mechanism of bone mineralization. Cold Spring Harb. Perspect Med. 8: a031229. https://doi.10.1101/cshperspect.a031229.
21. Laurencin D, et al. (2011) Magnesium incorporation into hydroxyapatite. Biomaterials 32: 1826-1837. https://doi.org/10.1016/j.biomaterials.2010.11.017.
22. Wu L, Luthringer BJ, Feyerabend F, Schilling AF, Willumeit R (2014) Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater 10: 2843-2854. https://doi.org/10.1016/j.actbio.2014.02.010.
23. Wu L, Feyerabend F, Schilling AF, Willumeit-Römer R, Luthringer BJC (2015) Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater 27: 294-304. https://doi.org/10.1016/j.actbio.2015.08.042.
24. Dimitrov V, White JH (2017) Vitamin D signaling in intestinal innate immunity and homeostasis. Mol. Cell. Endocrinol. 453: 68-78. https://doi.org/10.1016/j.mce.2017.04.010.
25. Glowka E, Stasiak J, Lulek J (2019) Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics 11: 347. https://doi.org/10.3390/pharmaceutics11070347.
26. Fang N, et al. (2019) Identification of a novel melatonin-binding nuclear receptor: Vitamin D receptor. J. Pineal Res. 68: e12618. https://doi.org/10.1111/jpi.12618.
27. Amstrup AK, et al. (2015) Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: A randomized controlled trial. J. Pineal Res. 59: 221-229. https://doi.org/10.1111/jpi.12252.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


