Melatonin, tunneling nanotubes and anastasis: Cheating cell death
Melatonin and anastasis
Abstract
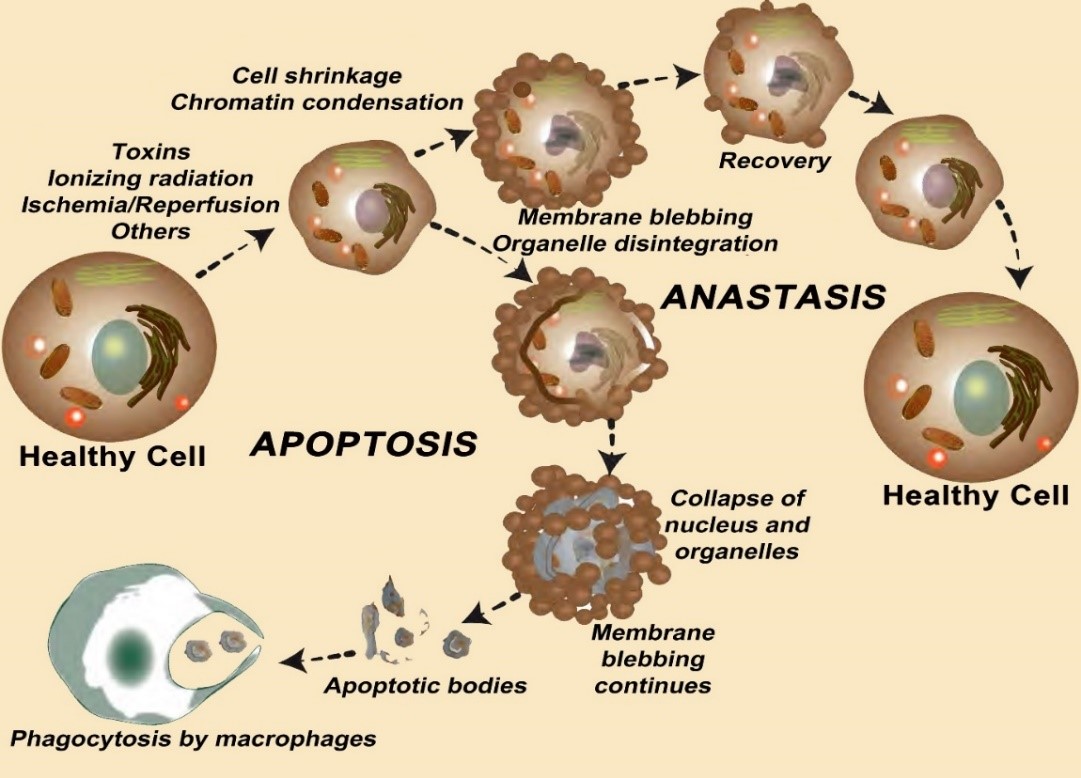
When healthy neurons are exposed to toxins or physiological insults such as ischemia, apoptosis is often initiated. Once underway, this mechanistically-well described process was thought to routinely run its course with the disintegration of the cell and phagocytosis of the debris. Within the last decade, the consistency of this process has been questioned. It is now known that some damaged cells can recover, i.e., they avoid death; this restoration process is referred to as anastasis. The reestablishment of a healthy cell phenotype is highly energy-requiring, so optimally functioning mitochondria are obviously beneficial during the regenerative process. Some healthy mitochondria that end up in regenerating cells are transferred there by adjacent healthier cells through tunneling nanotubes. Tunneling nanotubes generally form under stressful conditions when these micron-size tubules link adjacent cells. These tubules transfer soluble factors and organelles, including mitochondria, between the connected cells. When damaged cells receive high APT-producing mitochondria via this means, they support the ability of the cells to recover. Two recent comprehensive publications show that melatonin aids the transfer of mitochondria through nanotubes that connect neurons thereby likely assisting the recovery of the damaged recipient cell. Thus, melatonin not only protects normal neurons from damage by neutralizing the agents that initiate apoptosis, e.g., free radicals, etc., but also reverses this process once it is underway.References
2. Tang HL, Tang HM, Mak KH, Hu S, Wang SS, Wong KM, Wong CS, Wu HY, Law HT, Liu K, Talbot CC, Jr., Lau WK, Montell DJ, Fung MC (2012) Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol. Biol. Cell 23 (12):2240-2252. doi:10.1091/mbc.E11-11-0926.
3. Haider N, Narula N, Narula J (2002) Apoptosis in heart failure represents programmed cell survival, not death, of cardiomyocytes and likelihood of reverse remodeling. J. Card. Fail. 8 (6 Suppl): S512-517. doi:10.1054/jcaf.2002.130034.
4. Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26 (4): 239-257. doi:10.1038/bjc.1972.33.
5. Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35 (4):.495-516. doi:10.1080/01926230701320337.
6. Dou L, Jourde-Chiche N (2019) Endothelial toxicity of high glucose and its by-products in diabetic kidney disease. Toxins (Basel) 11 (10): 578. doi:10.3390/toxins11100578.
7. Joffre J, Hellman J, Ince C, Ait-Oufella H (2020) Endothelial responses in sepsis. Am. J. Respir. Crit. Care Med. 202 (3): 361-370. doi:10.1164/rccm.201910-1911TR.
8. Shukla M, Htoo HH, Wintachai P, Hernandez JF, Dubois C, Postina R, Xu H, Checler F, Smith DR, Govitrapong P, Vincent B (2015) Melatonin stimulates the nonamyloidogenic processing of betaAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J. Pineal Res. 58 (2): 151-165. doi:10.1111/jpi.12200.
9. Loh D, Reiter RJ (2021) Melatonin: regulation of biomolecular condensates in neurodegenerative disorders. Antioxidants (Basel) 10 (9): 1483. doi:10.3390/antiox10091483.
10. Churchill MJ, Mitchell PS, Rauch I (2021) Epithelial pyroptosis in host defense. J. Mol. Biol. 167278. doi:10.1016/j.jmb.2021.167278.
11. Bagayoko S, Meunier E (2021) Emerging roles of ferroptosis in infectious diseases. FEBS J. doi:10.1111/febs.16244.
12. Thomas CN, Berry M, Logan A, Blanch RJ, Ahmed Z (2017) Caspases in retinal ganglion cell death and axon regeneration. Cell Death Discov 3:17032. doi:10.1038/cddiscovery.2017.32.
13. Aryaman J, Johnston IG, Jones NS (2018) Mitochondrial heterogeneity. Front Genet. 9: 718. doi:10.3389/fgene.2018.00718.
14. Tang HM, Tang HL (2018) Anastasis: recovery from the brink of cell death. R. Soc. Open Sci. 5 (9): 180442. doi:10.1098/rsos.180442.
15. Pulkki AS, Voipio-Pulkki LM (2000) Significance of myocytes with positive DNA in situ nick end-labeling (TUNEL) in hearts with dilated cardiomyopathy. Circulation 101 (25): E239. doi:10.1161/01.cir.101.25.e239.
16. Zaitceva V, Kopeina GS, Zhivotovsky B (2021) Anastasis: Return journey from cell death. Cancers (Basel) 13 (15): 3671. doi:10.3390/cancers13153671.
17. Bhola PD, Letai A (2016) Mitochondria-judges and executioners of cell death sentences. Mol. Cell 61 (5): 695-704. doi:10.1016/j.molcel.2016.02.019.
18. Riedl SJ, Shi Y (2004) Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol Cell Biol. 5 (11): 897-907. doi:10.1038/nrm1496.
19. Neame SJ, Rubin LL, Philpott KL (1998) Blocking cytochrome c activity within intact neurons inhibits apoptosis. J. Cell Biol. 142 (6): 1583-1593. doi:10.1083/jcb.142.6.1583.
20. Tang HL, Tang HM, Hardwick JM, Fung MC (2015) Strategies for tracking anastasis, a cell survival phenomenon that reverses apoptosis. J. Vis. Exp. 96: e53964. doi:10.3791/51964.
21. Baranov SV, Baranova OV, Yablonska S, Suofu Y, Assquez AL, Kozai TDY, Cui XT, Ferranndo LM, Larkin TM, Tyurina YY, Kagan VE, Carlisle DL, Kristal BS, Friedlander RM. (2019) Mitochodria modulate programmed neurite retraction. Proc. Nat. Acad. Sci. USA 116 (2): 650-659. doi: 10.1073/pnas.1811021116.
22. Baranov SV, Jauhari A, Carlisle DL, Friedlander RM (2021) Two hit mitochondrail-driven model of synapse loss in neurodegeneration. Neurobiol. Dis. 158: 105451. doi: 10.1016/j.nbd.2021.105451.
23. Tait SW, Parsons MJ, Llambi F, Bouchier-Hayes L, Connell S, Munoz-Pinedo C, Green DR (2010) Resistance to caspase-independent cell death requires persistence of intact mitochondria. Dev. Cell 18 (5): 802-813. doi:10.1016/j.devcel.2010.03.014.
24. Mirzayans R, Murray D (2020) Intratumor heterogeneity and therapy resistance: contributions of dormancy, apoptosis reversal (anastasis) and cell fusion to disease recurrence. Int. J. Mol. Sci. 21 (4): 1308.. doi:10.3390/ijms21041308.
25. He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G (2016) Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte's quality under in vitro conditions. Int. J. Mol. Sci. 17 (6): 939. doi:10.3390/ijms17060939.
26. Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y, Cecon E, Wehbi VL, Kim J, Heath BE, Baranova OV, Wang X, Gable MJ, Kretz ES, Di Benedetto G, Lezon TR, Ferrando LM, Larkin TM, Sullivan M, Yablonska S, Wang J, Minnigh MB, Guillaumet G, Suzenet F, Richardson RM, Poloyac SM, Stolz DB, Jockers R, Witt-Enderby PA, Carlisle DL, Vilardaga JP, Friedlander RM (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U S A 114 (38): E7997-E8006. doi:10.1073/pnas.1705768114.
27. Reiter RJ, Sharma R, Ma Q, Rosales-Corral S, Acuna-Castroviejo D, Escames G (2019) Inhibition of mitochondrial pyruvate dehydrogenase kinase: a proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2 (3): 105-119.
28. Reiter RJ, Sharma R, Rodriguez C, Martin V, Rosales-Corral S, Zuccari D, Chuffa LGA (2021) Part-time cancers and role of melatonin in determining their metabolic phenotype. Life Sci. 278:119597. doi:10.1016/j.lfs.2021.119597.
29. Reiter RJ, Sharma R, Ma Q (2021) Switching diseased cells from cytosolic aerobic glycolysis to mitochondrial oxidative phosphorylation: A metabolic rhythm regulated by melatonin? J. Pineal Res. 70 (1):e12677. doi:10.1111/jpi.12677.
30. Chen X, Hao B, Li D, Reiter RJ, Bai Y, Abay B, Chen G, Lin S, Zheng T, Ren Y, Xu X, Li M, Fan L (2021) Melatonin inhibits lung cancer development by reversing the Warburg effect via stimulating the SIRT3/PDH axis. J. Pineal Res. e: 12755. doi:10.1111/jpi.12755.
31. Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, Frasch T, Blask DE (2015) Melatonin: An inhibitor of breast cancer. Endocr. Rela.t Cancer 22 (3): R183-204. doi:10.1530/ERC-15-0030.
32. de Almeida Chuffa LG, Seiva FRF, Cucielo MS, Silveira HS, Reiter RJ, Lupi LA (2019) Mitochondrial functions and melatonin: a tour of the reproductive cancers. Cell Mol. Life Sci. 76 (5): 837-863. doi:10.1007/s00018-018-2963-0.
33. Pan S, Guo Y, Hong F, Xu P, Zhai Y (2021) Therapeutic potential of melatonin in colorectal cancer: Focus on lipid metabolism and gut microbiota. Biochim. Biophys. Acta. Mol. Basis Dis. 1868 (1): 166281. doi:10.1016/j.bbadis.2021.166281.
34. Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH (2004) Nanotubular highways for intercellular organelle transport. Science 303 (5660): 1007-1010. doi:10.1126/science.1093133.
35. Korenkova O, Pepe A, Zurzolo C (2020) Fine intercellular connections in development: TNTs, cytonemes, or intercellular bridges? Cell Stress 4 (2): 30-43. doi:10.15698/cst2020.02.212.
36. Schiller C, Diakopoulos KN, Rohwedder I, Kremmer E, von Toerne C, Ueffing M, Weidle UH, Ohno H, Weiss EH (2013) LST1 promotes the assembly of a molecular machinery responsible for tunneling nanotube formation. J. Cell Sci. 126 (Pt 3): 767-777. doi:10.1242/jcs.114033.
37. Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A (2014) Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 33 (9): 994-1010. doi:10.1002/embj.201386030.
38. Vignais ML, Caicedo A, Brondello JM, Jorgensen C (2017) Cell connections by tunneling nanotubes: effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int. 2017: 6917941. doi:10.1155/2017/6917941.
39. MacAskill AF, Kittler JT (2010) Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 20 (2): 102-112. doi:10.1016/j.tcb.2009.11.002.
40. Wang X, Gerdes HH (2015) Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ 22 (7): 1181-1191. doi:10.1038/cdd.2014.211.
41. Diaz-Casado ME, Rusanova I, Aranda P, Fernandez-Ortiz M, Sayed RKA, Fernandez-Gil BI, Hidalgo-Gutierrez A, Escames G, Lopez LC, Acuna-Castroviejo D (2018) In vivo determination of mitochondrial respiration in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated zebrafish reveals the efficacy of melatonin in restoring mitochondrial normalcy. Zebrafish 15 (1): 15-26. doi:10.1089/zeb.2017.1479.
42. Dupont M, Souriant S, Lugo-Villarino G, Maridonneau-Parini I, Verollet C (2018) Tunneling nanotubes: Intimate communication between myeloid cells. Front Immunol. 9: 43. doi:10.3389/fimmu.2018.00043.
43. Osswald M, Jung E, Wick W, Winkler F (2019) Tunneling nanotube-like structures in brain tumors. Cancer Rep. 2 (6): e1181. doi:10.1002/cnr2.1181.
44. Hashimoto M, Bhuyan F, Hiyoshi M, Noyori O, Nasser H, Miyazaki M, Saito T, Kondoh Y, Osada H, Kimura S, Hase K, Ohno H, Suzu S (2016) Potential role of the formation of tunneling nanotubes in HIV-1 spread in macrophages. J. Immunol. 196 (4): 1832-1841. doi:10.4049/jimmunol.1500845.
45. Victoria GS, Zurzolo C (2017) The spread of prion-like proteins by lysosomes and tunneling nanotubes: Implications for neurodegenerative diseases. J. Cell Biol. 216 (9): 2633-2644. doi:10.1083/jcb.201701047.
46. Austefjord MW, Gerdes HH, Wang X (2014) Tunneling nanotubes: Diversity in morphology and structure. Commun. Integr. Biol. 7 (1): e27934. doi:10.4161/cib.27934.
47. Yamashita YM, Inaba M, Buszczak M (2018) Specialized intercellular communications via cytonemes and nanotubes. Annu. Rev. Cell Dev. Biol. 34: 59-84. doi:10.1146/annurev-cellbio-100617-062932.
48. Torralba D, Baixauli F, Sanchez-Madrid F (2016) Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front Cell Dev. Biol. 4: 107. doi:10.3389/fcell.2016.00107.
49. Zampieri LX, Silva-Almeida C, Rondeau JD, Sonveaux P (2021) Mitochondrial transfer in cancer: a comprehensive review. Int. J. Mol. Sci. 22 (6): 487-514, doi:10.3390/ijms22063245.
50. Pegtel DM, Gould SJ (2019) Exosomes. Annu. Rev. Biochem. 88: 487-514. doi:10.1146/annurev-biochem-013118-111902.
51. Novais AA, Chuffa LGA, Zuccari D, Reiter RJ (2021) Exosomes and melatonin: Where their destinies intersect. Front Immunol. 12: 692022. doi:10.3389/fimmu.2021.692022.
52. Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C (2014) Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 92: 10-18. doi:10.1016/j.mvr.2014.01.008.
53. Soundara Rajan T, Gugliandolo A, Bramanti P, Mazzon E (2020) Tunneling nanotubes-mediated protection of mesenchymal stem cells: An update from preclinical studies. Int. J. Mo.l Sci. 21 (10): 3481. doi:10.3390/ijms21103481.
54. Gomzikova MO, James V, Rizvanov AA (2021) Mitochondria donation by mesenchymal stem cells: current understanding and mitochondria transplantation strategies. Front Cell Dev. Biol. 9: 653322. doi:10.3389/fcell.2021.653322.
55. Liu CS, Chang JC, Kuo SJ, Liu KH, Lin TT, Cheng WL, Chuang SF (2014) Delivering healthy mitochondria for the therapy of mitochondrial diseases and beyond. Int. J. Biochem. Cell Biol. 53:.141-146. doi:10.1016/j.biocel.2014.05.009.
56. Han H, Hu J, Yan Q, Zhu J, Zhu Z, Chen Y, Sun J, Zhang R (2016) Bone marrow-derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Mol. Med. Rep. 13 (2): 1517-1524. doi:10.3892/mmr.2015.4726.
57. Li CJ, Chen PK, Sun LY, Pang CY (2017) Enhancement of mitochondrial transfer by antioxidants in human mesenchymal stem cells. Oxid. Med. Cell Longev. 2017: 8510805. doi:10.1155/2017/8510805.
58. Liu K, Guo L, Zhou Z, Pan M, Yan C (2019) Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc. Res. 123: 74-80. doi:10.1016/j.mvr.2019.01.001.
59. Ma Z, Xin Z, Di W, Yan X, Li X, Reiter RJ, Yang Y (2017) Melatonin and mitochondrial function during ischemia/reperfusion injury. Cell Mol. Life Sci. 74 (21): 3989-3998. doi:10.1007/s00018-017-2618-6.
60. Vongsfak J, Pratchayasakul W, Apaijai N, Vaniyapong T, Chattipakorn N, Chattipakorn SC (2021) The alterations in mitochondrial dynamics following cerebral ischemia/reperfusion injury. Antioxidants (Basel) 10 (9): 1384. doi:10.3390/antiox10091384.
61. Liu K, Guo L, Zhou Z, Pan M, Yan C. (2019) Mesenchymal stem cells transfer mitochondria into cerebral microvascular and promote recovery from ischemic stroke. Microvasc. Res. 123:7 4-80. doi:10.1016/j.mvr.2019.01.001.
62. Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535 (7613): 551-555. doi:10.1038/nature18928.
63. Agnati LF, Fuxe K (2014) Extracellular-vesicle type of volume transmission and tunnelling-nanotube type of wiring transmission add a new dimension to brain neuro-glial networks. Philos. Trans. R. Soc. Lond B Biol. Sci. 369 (1652): 2130505. doi:10.1098/rstb.2013.0505.
64. Valdinocci D, Simoes RF, Kovarova J, Cunha-Oliveira T, Neuzil J, Pountney DL (2019) Intracellular and intercellular mitochondrial dynamics in parkinson's disease. Front Neurosci. 13: 930. doi:10.3389/fnins.2019.00930.
65. Zurzolo C (2021) Tunneling nanotubes: Reshaping connectivity. Curr. Opin. Cell Biol. 71: 139-147. doi:10.1016/j.ceb.2021.03.003.
66. Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF, Xu K (2017) Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 18 (4): 843. doi:10.3390/ijms18040843.
67. Wu HJ, Wu C, Niu HJ, Wang K, Mo LJ, Shao AW, Dixon BJ, Zhang JM, Yang SX, Wang YR (2017) Neuroprotective mechanisms of melatonin in hemorrhagic stroke. Cell Mol. Neurobiol. 37 (7):1173-1185. doi:10.1007/s10571-017-0461-9.
68. Ramos E, Patino P, Reiter RJ, Gil-Martin E, Marco-Contelles J, Parada E, de Los Rios C, Romero A, Egea J (2017) Ischemic brain injury: New insights on the protective role of melatonin. Free Radic. Biol. Med. 104: 32-53. doi:10.1016/j.freeradbiomed.2017.01.005.
69. Yawoot N, Govitrapong P, Tocharus C, Tocharus J (2021) Ischemic stroke, obesity, and the anti-inflammatory role of melatonin. Biofactors 47 (1): 41-58. doi:10.1002/biof.1690.
70. Lochner A, Marais E, Huisamen B (2018) Melatonin and cardioprotection against ischaemia/reperfusion injury: What's new? A review. J. Pineal Res. 65 (1): e12490. doi:10.1111/jpi.12490.
71. Dominguez-Rodriguez A, Abreu-Gonzalez P, Baez-Ferrer N, Reiter RJ, Avanzas P, Hernandez-Vaquero D (2021) Melatonin and cardioprotection in humans: a systematic review and meta-analysis of randomized controlled trials. Front Cardiovasc. Med. 8: 635083. doi:10.3389/fcvm.2021.635083.
72. Jurcau A, Ardelean IA (2021) Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. J. Integr. Neurosci 20 (3): 727-744. doi:10.31083/j.jin2003078.
73. Huo X, Wang C, Yu Z, Peng Y, Wang S, Feng S, Zhang S, Tian X, Sun C, Liu K, Deng S, Ma X (2017) Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 62 (4): e12390. doi:10.1111/jpi.12390.
74. Mayo JC, Sainz RM, Gonzalez-Menendez P, Hevia D, Cernuda-Cernuda R (2017) Melatonin transport into mitochondria. Cell Mol. Life Sci. 74 (21): 3927-3940. doi:10.1007/s00018-017-2616-8.
75. Kopustinskiene DM, Bernatoniene J (2021) Molecular mechanisms of melatonin-mediated cell protection and signaling in health and disease. Pharmaceutics 13 (2): 129. doi:10.3390/pharmaceutics13020129.
76. Melhuish Beaupre LM, Brown GM, Goncalves VF, Kennedy JL (2021) Melatonin's neuroprotective role in mitochondria and its potential as a biomarker in aging, cognition and psychiatric disorders. Transl. Psychiatry 11 (1): 339. doi:10.1038/s41398-021-01464-x.
77. Tan HY, Ng KY, Koh RY, Chye SM (2020) Pharmacological effects of melatonin as neuroprotectant in rodent model: A review on the current biological evidence. Cell Mol. Neurobiol. 40 (1): 25-51. doi:10.1007/s10571-019-00724-1.
78. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61 (3): 253-278. doi:10.1111/jpi.12360.
79. Hardeland R (2018) Melatonin and inflammation-story of a double-edged blade. J. Pineal Res. 65 (4): e12525. doi:10.1111/jpi.12525.
80. Chitimus DM, Popescu MR, Voiculescu SE, Panaitescu AM, Pavel B, Zagrean L, Zagrean AM (2020) Melatonin's impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules 10 (9): 1211. doi:10.3390/biom10091211.
81. Yip HK, Dubey NK, Lin KC, Sung PH, Chiang JY, Chu YC, Huang CR, Chen YL, Deng YH, Cheng HC, Deng WP (2021) Melatonin rescues cerebral ischemic events through upregulated tunneling nanotube-mediated mitochondrial transfer and downregulated mitochondrial oxidative stress in rat brain. Biomed. Pharmacother. 139: 111593. doi:10.1016/j.biopha.2021.111593.
82. Nasoni MG, Carloni S, Canonico B, Burattini S, Cesarini E, Papa S, Pagliarini M, Ambrogini P, Balduini W, Luchetti F (2021) Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J. Pineal Res. 71 (1): e12747. doi:10.1111/jpi.12747.
83. Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 17 (12): 2124. Doi: 10.3390/ijms17122124.
84. Wang A, Zhao Z, Feng X, Cheng Z, Xiong Z, Wang T, Lin J, Hu J, Fan Y, Reiter RJ, Wang H, Sun D. (2018) Melatonin activates Parkin translocation and rescuses the impaired mitophagy activity of diabetic cardiomyopathy through Mst1 inhibition. J. Cell Mol. Med. 22 (10): 5132-5144. doi: 10.1111/jcmm.13802.
85. Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, Garcia-Corzo L, Lopez LC, Reiter RJ, Acuna-Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52 (2): 217-227. doi:10.1111/j.1600-079X.2011.00931.x.
86. Acuña-Castroviejo D, Noguiera-Navarro M, Reiter R, Escames G (2018) Melatonin actions in the heart; more than a hormone. Melatonin Res. 1: 21-26.
87. Swenson ER (2016) Hypoxia and its acid-base consequences: from mountains to malignancy. Adv. Exp. Med. Biol. 903: 301-323. doi:10.1007/978-1-4899-7678-9_21.
88. Reiter RJ, Sharma R, Rosales-Corral S (2021) Anti-warburg effect of melatonin: a proposed mechanism to explain its inhibition of multiple diseases. Int. J. Mol. Sci. 22 (2): 764. doi:10.3390/ijms22020764.
89. Martı́n M, Macı́as M, León J, Escames G, Khaldy H, Acuña-Castroviejo D (2002) Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell Biol. 34 (4): 348-357. doi:https://doi.org/10.1016/S1357-2725(01)00138-8.
90. Acuna Castroviejo D, Lopez LC, Escames G, Lopez A, Garcia JA, Reiter RJ (2011) Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 11 (2): 221-240. doi:10.2174/156802611794863517.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


