An insight into the ameliorative effects of melatonin against chromium induced oxidative stress and DNA damage: a review
Melatonin attenuates chromium-induced oxidative damage
Abstract
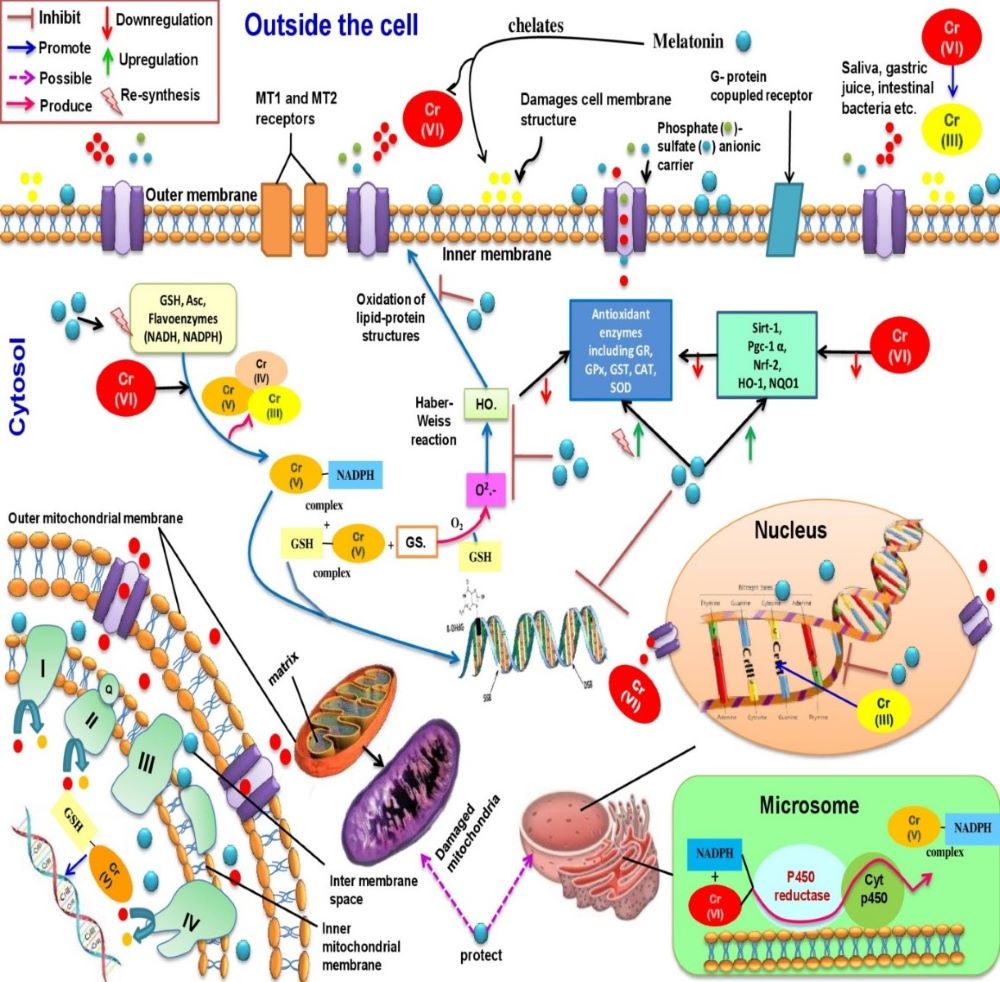
Chromium (Cr), a ubiquitous metal, has become a potent pollutant due to global industrialization, leading to pollution of air, water, and food that impacts human health. The most stable forms of Cr are Cr(III) and Cr(VI) (the major product of industrial activities). Cr(III) is a micronutrient essential for maintaining normal blood glucose and lipid profiles in our body but it can also form Cr (III)-DNA adducts. In addition, it directly produces reactive oxygen species (ROS) via Fenton and Haber-Weiss reactions; leading to tissue injuries. Cr (VI) has the capacity to generate Cr(V), Cr (IV), and Cr(III), respectively under suitable conditions. These intermediates also damage to biological macromolecules by interactions with several enzymatic and non-enzymatic antioxidants. For example, Cr(III) can make double DNA strands breaking to inhibit DNA replication, induce DNA oxidation, and DNA adducts formation. All of these lead to the development of malignancy. Melatonin, a potent radical scavenger as well as a metal chelator, effectively chelates Cr(VI) and prevents DNA oxidative damage. Melatonin can upregulate the gene expression of several antioxidant enzymes, and thereby, maintains cellular integrity from the oxidative stress. Thus, melatonin can be a prime molecule to protect against Cr(VI) induced cytotoxicity and genotoxicity. This review aims to highlight the potential benefits of melatonin on Cr(VI) induced oxidative stress and DNA damage.
References
2. Rita Evelyne J, Ravisankar V (2014) Bioremediation of chromium contamination- a review. Int. J. Res. Earth Environ. Sci. 1 (6): 20–26.
3. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 101: 133–164. DOI: 10.1007/978-3-7643-8340-4.
4. Katz SA, Salem H (1993) The toxicology of chromium with respect to its chemical speciation: A review. J. Appl. Toxicol. 13 (3): 217–224. DOI: 10.1002/jat.2550130314.
5. Goyer RA, Clarkson TW 2001. (2001) Toxic effects of metals. In: Klaassen CD (ed) Cassarett and Doull’s toxicology: the basic science of poisons McGraw-Hill, New York. 2001. p. 811–867.
6. Duffus JH (2002) “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 74 (5): 793–807. DOI: 10.1351/pac200274050793.
7. Schmidt RL (1984) Thermodynamic properties and environmental chemistry of chromium. Richland, WA; 1984.
8. Barnhart J (1997) Occurrences, uses, and properties of chromium. Regul. Toxicol. Pharmacol. 26 (1): S3–S7. DOI: 10.1006/rtph.1997.1132.
9. Mertz W (1969) Chromium occurrence and function in biological systems. Physiol. Rev. 49 (2): 163–239. DOI: 10.1152/physrev.1969.49.2.163.
10. Anderson RA, Polansky MM, Bryden NA, Roginski EE, Mertz W, Glinsmann W (1983) Chromium supplementation of human subjects: Effects on glucose, insulin, and lipid variables. Metabolism 32 (9): 894–899. DOI: 10.1016/0026-0495(83)90203-2.
11. Anderson RA, Polansky MM, Bryden NA, Canary JJ (1991) Supplemental-chromium effects on glucose, insulin, glucagon, and urinary chromium losses in subjects consuming controlled low-chromium diets. Am. J. Clin. Nutr. 54 (5): 909–916.
12. Mertz W (1993) Chromium in human nutrition: A review. J. Nutr. 123 (4): 626–633. DOI: 10.1093/jn/123.4.626.
13. Pechova A, Pavlata L (2007) Chromium as an essential nutrient: a review. Vet. Med. (Praha). 52 (1): 1–18. DOI: 10.17221/2010-VETMED.
14. Abraham SA, Baruch AB EU (1991) Chromium and cholesterol-induced atherosclerosis in rabbits. Ann. Nutr. Metab. 35: 203–207. DOI: Doi: 10.1159/000177646.
15. Abraham AS, Brooks BA, Eylath U (1992) The effects of chromium supplementation on serum glucose and lipids in patients with and without non-insulin-dependent diabetes. Metabolism 41 (7): 768–771. DOI: 10.1016/0026-0495(92)90318-5.
16. Balk E, Tatsioni A, Lichtenstein A, Lau J, Pittas A (2007) Effect of chromium supplementation on glucose metabolism and lipids. Diabetes Care 30 (8): 2154–2163. DOI: Doi: 10.2337/dc06-0996.
17. Davis CM, Vincent JB (1997) Chromium in carbohydrate and lipid metabolism. J. Biol. Inorg. Chem. 2 (6): 675–679.
18. Anderson RA (1998) Chromium, glucose intolerance and diabetes. J. Am. Coll. Nutr. 17 (6): 548–555. DOI: 10.1080/07315724.1998.10718802.
19. Saha R, Nandi R, Saha B (2011) Sources and toxicity of hexavalent chromium. J. Coord. Chem. 64 (10): 1782–1806. DOI: 10.1080/00958972.2011.583646.
20. Kimbrough DE, Cohen Y, Winer AM, Creelman L, Kimbrough DE (1999) A critical assessment of chromium in the environment. Crit. Rev. Environ. Sci. Technol. 29 (1): 1–46. DOI: 10.1080/10643389991259164.
21. Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Heal. - Part C Environ. Carcinog. Ecotoxicol. Rev. 34 (1): 1–32. DOI: 10.1080/10590501.2015.1096883.
22. Shrivastava R, Upreti RK, Chaturvedi UC (2003) Various cells of the immune system and intestine differ in their capacity to reduce hexavalent chromium. FEMS Immunol. Med. Microbiol. 38 (1): 65–70. DOI: 10.1016/S0928-8244(03)00107-X.
23. Chuan MC, Liu JC (1996) Release behavior of chromium from tannery sludge. Water Res. 30 (4): 932–938. DOI: 10.1016/0043-1354(95)00227-8.
24. ATSDR (2012) Public health statement: Chromium. Public Heal. Serv. (September): 1–9.
25. Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part i: mechanisms involved in metal induced oxidative damage. Curr. Top. Med. Chem. 1 (6): 529–539. DOI: 10.2174/1568026013394831.
26. Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 18 (2): 321–336. DOI: 10.1016/0891-5849(94)00159-H.
27. Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res. Int. : 1–26. DOI: 10.1155/2014/640754.
28. Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radic. Biol. Med. 37 (12): 1921–1942. DOI: Doi:10.1016/j.freeradbiomed.2004.09.010.
29. Valko M, Morris H and CM (2005) Metals, toxicity and oxidative stress. Curr. Med. Chem. 12 : 1161–1208. DOI: Doi: 10.2174/0929867053764635.
30. Soudani N, Troudi A, Bouaziz H, Ben Amara I, Boudawara T, Zeghal N (2011) Cardioprotective effects of selenium on chromium (VI)-induced toxicity in female rats. Ecotoxicol. Environ. Saf. 74 (3): 513–520. DOI: 10.1016/j.ecoenv.2010.06.009.
31. Soudani N, Ben Amara I, Sefi M, Boudawara T, Zeghal N (2011) Effects of selenium on chromium (VI)-induced hepatotoxicity in adult rats. Exp. Toxicol. Pathol. 63 (6): 541–548. DOI: 10.1016/j.etp.2010.04.005.
32. Balakrishnan R, Satish Kumar CSV, Rani MU, Srikanth MK, Boobalan G, Reddy AG (2013) An evaluation of the protective role of α-tocopherol on free radical induced hepatotoxicity and nephrotoxicity due to chromium in rats. Indian J. Pharmacol. 45 (5): 490–495. DOI: 10.4103/0253-7613.117778.
33. Saidi M, Aouacheri O, Saka S (2019) Protective effect of curcuma against chromium hepatotoxicity in rats. Phytothérapie 18 (3–4): 148–155. DOI: 10.3166/phyto-2019-0114.
34. Soudani N, Sefi M, Ben Amara I, Boudawara T, Zeghal N (2010) Protective effects of selenium (Se) on chromium (VI) induced nephrotoxicity in adult rats. Ecotoxicol. Environ. Saf. 73 (4): 671–678. DOI: 10.1016/j.ecoenv.2009.10.002.
35. Lanca S, Alves A, Vieira AI, Barata J, Freitas JD and CA (2002) Chromium-induced toxic hepatitis. Eur. J. Intern. Med. 13 : 518–520. DOI: Https://doi.org/10.1016/S0953-6205(02)00164-4.
36. Shrivastava R, Upreti RK, Seth PK, Chaturvedi UC (2002) Effects of chromium on the immune system. FEMS Immunol. Med. Microbiol. 34 (1): 1–7. DOI: 10.1016/S0928-8244(02)00345-0.
37. Burastero SE, Paolucci C, Breda D, Pontp J MB and SE (2006) Chromium (VI)-induced immunotoxicity and intracellular accumulation in human primary dendritic cells. Int. J. Immunopathol. Pharmacol. 19 (3): 581–591.
38. Nickens KP, Patierno SR, Ceryak S (2010) Chromium genotoxicity: A double-edged sword. Chem. Biol. Interact. 188 (2): 276–288. DOI: 10.1016/j.cbi.2010.04.018.
39. Blasiak J, Kowalik J (2000) A comparison of the in vitro genotoxicity of tri- and hexavalent chromium. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 469 (1): 135–145. DOI: 10.1016/S1383-5718(00)00065-6.
40. Bianchi V, Levis AG (1984) Mechanisms of chromium genotoxicity. Toxicol. Environ. Chem. 9 (1): 1–25. DOI: 10.1080/02772248409357064.
41. Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ. Int. 31 (5): 739–753.
42. Oliveira H (2012) Chromium as an environmental pollutant: Insights on induced plant toxicity. J. Bot. 2012 : 1–8.
43. Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, Jasrotia S, Zheng B, Yuan H, Yan D (2020) Chromium bioaccumulation and its impacts on plants: An overview. Plants. 9 (1): 1–17.
44. Kundu D, Dey S Raychaudhuri SS (2018) Chromium (VI) - induced stress response in the plant Plantago ovata Forsk in vitro. Genes Environ. 40 (1): 1–13.
45. Stambulska UY, Bayliak MM, Lushchak VI (2018) Chromium(VI) toxicity in legume plants: Modulation effects of rhizobial symbiosis. Biomed. Res. Int. 2018: 8031213. doi: 10.1155/2018/8031213.
46. Seleiman MF, Ali S, Refay Y, Rizwan M, Alhammad BA, El-Hendawy SE (2020) Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 250:126239. DOI: 10.1016/j.chemosphere.2020.126239.
47. Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 178 : 513–533.
48. Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 34 (1): 75–78. DOI: 10.1034/j.1600-079X.2003.02111.x.
49. Reiter RJ (2000) Melatonin: Lowering the high price of free radicals. News Physiol. Sci. 15 (5): 246–250. DOI: 10.1152/physiologyonline.2000.15.5.246.
50. Reiter RJ (2003) Melatonin: clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab. 17 (2): 273–285. DOI: 10.1053/ybeem.2003.249.
51. Poeggeler B RR, Tan DX, Chen LD and ML (1993) Melatonin, hydroxyl radical-mediated oxidative damage , and aging : A hypothesis. J. Pineal Res. 14 : 151–168. DOI: DOI: 10.1111/j.1600-079x.1993.tb00498.x.
52. Cardinali DP, Srinivasan V, Brzezinski A, Brown GM (2011) Melatonin and its analogs in insomnia and depression. J. Pineal Res. 52 (4): 1–11. DOI: 10.1111/j.1600-079X.2011.00962.x.
53. Chen CQ, Fichna J, Bashashati M, Li YY, Storr M (2011) Distribution, function and physiological role of melatonin in the lower gut. World J. Gastroenterol. 17 (34): 3888–3898.
54. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 71 (16): 2997–3025.
55. Galano A, Tan DX, Reiter RJ (2012) On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 54 (3): 245–257. DOI: 10.1111/jpi.12010.
56. Hardeland R (2005) Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 27 (2): 119–130. DOI: 10.1385/ENDO:27:2:119.
57. Hardeland R, Reiter RJ, Poeggeler B, Tan DX (1993) The significance of the metabolism of the neurohormone melatonin: Antioxidative protection and formation of bioactive substances. Neurosci. Biobehav. Rev. 17 (3): 347–357. DOI: 10.1016/S0149-7634(05)80016-8.
58. Romero A, Ramos E, De Los Ríos C, Egea J, Del Pino J, Reiter RJ (2014) A review of metal-catalyzed molecular damage: Protection by melatonin. J. Pineal Res. 56 (4): 343–370. DOI: 10.1111/jpi.12132.
59. Rana SVS (2018) Protection of metal toxicity by melatonin - recent advances. EC Pharmacol. Toxicol. 9: 851–864.
60. Gulcin I, Buyukokuroglu ME, Kufrevioglu OI (2003) Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 34: 278–281. DOI: 10.1034/j.1600-079X.2003.00042.x.
61. Sun FY, Lin X, Mao LZ, Ge WH, Zhang LM, Huang YL, Gu J (2002) Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J. Pineal Res. 33 (1): 48–56.
62. Tarocco A, Caroccia N, Morciano G, Wieckowski MR, Ancora G, Garani G, Pinton P (2019) Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 10 (4): 317 DOI: 10.1038/s41419-019-1556-7.
63. Waseem M, Tabassum H, Parvez S (2016) Melatonin modulates permeability transition pore and 5-hydroxydecanoate induced KATP channel inhibition in isolated brain mitochondria. Mitochondrion 31: 1–8. DOI: 10.1016/j.mito.2016.08.005.
64. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J (2013) A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 54 (1): 1–14.
65. Cao Z, Fang Y, Lu Y, Tan DX, Du C, Li Y, Ma Q, Yu J, Chen M, Zhou C, Pei L, Zhang L, Ran H, He M, Yu Z ZZ (2017) Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J. Pineal Res. 62 (3): doi: 10.1111/jpi.12389.
66. Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Ali S, Zhou W (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120: 154–164. DOI: 10.1016/j.chemosphere.2014.06.029.
67. Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 22 (14): 10669–10678.
68. Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, Ibrahim M, Gill RA, Khan MD (2015) Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res. 22 (14): 10601–10609.
69. Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (2012) Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012: 577–597. DOI: 10.1093/jxb/err256.
70. Ayyaz A, Amir M, Umer S, Iqbal M, Bano H, Gul HS, Noor Y, kanwal A, khalid A, Javed M, Athar HR, Zafar ZU, Farooq MA (2020) Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 6 (7): e04364.
71. Marchiafava PL, Longoni B (1999) Melatonin as an antioxidant in retinal photoreceptors. J. Pineal Res. 26 (3): 184–189.
72. Bubenik GA, Pang SF (1994) The role of serotonin and melatonin in gastrointestinal physiology: Ontogeny, regulation of food intake, and mutual serotonin‐melatonin feedback. J. Pineal Res. 16 (2): 91–99.
73. Lee PPN, Shiu SYW, Chowb PH PS (1996) Regional and diurnal studies of melatonin and melatonin binding sites in the duck gastro-lntestinal tract. Biol. Signals. 4: 212–224.
74. Mojaverrostami S, Asghari N, Sc M, Khamisabadi M, Khoei HH, Abbas A (2019) The role of melatonin in polycystic ovary syndrome: A review. Int. J. Reprod. Biomed. 17 (12): 865–882.
75. Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee F, Tamura I, Maekawa R, Aasada H YY and SN (2012) The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 5 (5): 1–9.
76. Mertz W (1992) Chromium - History and nutritional importance. Biol. Trace Elem. Res. 32 (1–3): 3–8. DOI: 10.1007/BF02784581.
77. Vincent JB (2001) The bioinorganic chemistry of chromium(III). Polyhedron 20 : 1–26. DOI: 10.1016/S0277-5387(00)00624-0.
78. Vincent JB (2003) The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sport. Med. 33 (3): 213–230. DOI: 10.2165/00007256-200333030-00004.
79. Stearns DM, Joseph BJ, Wetterhahn EK (1995) A prediction humans of chromium ( III ) from accumulation dietary supplements in chromium. FASEB J. 9 (15): 1650–1657. DOI: Doi: 10.1096/fasebj.9.15.8529846.
80. Eastmond DA, MacGregor JT, Slesinski RS (2008) Trivalent chromium: Assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Crit. Rev. Toxicol. 38 (3): 173–190. DOI: 10.1080/10408440701845401.
81. Petrilli FL and Flora SD (1988) Metabolic reduction of chromium as a threshold mechanism limiting its in vivo activity. Sci. Total Environ. 71:357–364. DOI: 10.1016/0048-9697(88)90208-2.
82. Maret W (2019) Chromium supplementation in human health, metabolic syndrome, and diabetes. Met. Ions Life Sci. 19:231–251. DOI: 10.1515/9783110527872-015.
83. Katz SA (1991) The analytical biochemistry of chromium. Environ. Health Perspect. 92:13–16. DOI: 10.1289/ehp.919213.
84. Debetto P and Luciani S (1988) Toxic effect of chromium on cellular metabolism. Sci. Total Environ. 71:365–377. DOI: 10.1016/0048-9697(88)90209-4.
85. Chromium (VI) compounds. Vol. 100 C, IARC Monographs on the evaluation of carcinogenic risks to humans. 2011. p. 147–167.
86. Chiu A, Shi XL, Lee WKP, Hill R, Wakeman TP, Katz A, Xu B, Dalal NS, Robertson JD, Chen C, Chiu N, Donehower L (2010) Review of chromium (VI) apoptosis, cell-cycle-arrest, and carcinogenesis. J. Environ. Sci. Heal. - Part C Environ. Carcinog. Ecotoxicol. Rev. 28 (3): 188–230. DOI: 10.1080/10590501.2010.504980.
87. De Flora S, Serra D, Camoirano A, Zanacchi P (1989) Metabolic reduction of chromium, as related to its carcinogenic properties. Biol. Trace Elem. Res. 21 (1): 179–187. DOI: 10.1007/BF02917250.
88. Jennette KW (1979) Chromate metabolism in liver microsomes. Biol. Trace Elem. Res. 1 (1): 55–62. DOI: 10.1016/0006-291x(78)90931-2.
89. Costa M (1997) Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 27 (5): 431–442. DOI: 10.3109/10408449709078442.
90. Sun H, Brocato J, Costa M (2015) Oral chromium exposure and toxicity. Curr. Env. Heal. Rep. 2 (3): 295–303. DOI: 10.1007/s40572-015-0054-z.
91. Wang Y, Su H, Gu Y, Song X, Zhao J (2017) Carcinogenicity of chromium and chemoprevention: A brief update. Onco. Targets Ther. 10: 4065–4079. DOI: 10.2147/OTT.S139262.
92. De Flora S (2000) Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis 21 (4): 533–541. DOI: 10.1093/carcin/21.4.533.
93. Arslan P, Beltrame M, Tomasi A (1987) Intracellular chromium reduction. Biochim. Biophys. Acta - Mol. Cell Res. 931 (1): 10–15. DOI: 10.1016/0167-4889(87)90044-9.
94. O’Brien T, Xu J, Patierno SR (2001) Effects of glutathione on chromium-induced DNA crosslinking and DNA polymerase arrest. Mol. Cell. Biochem. 222 (1–2): 173–182. DOI: 10.1023/A:1017918330073.
95. Chen QY, Murphy A, Sun H CM (2019) Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol. Appl. Pharmacol. 377: DOI: 10.1016/j.taap.2019.114636.
96. Kitagawa S, Seki H, Kametani F, Sakurai H (1988) EPR study on the interaction of hexavalent chromium with glutathione or cysteine: Production of pentavalent chromium and its stability. Inorganica Chim. Acta. 152: 251–255. DOI: 10.1016/S0020-1693(00)91477-4.
97. Zhitkovich A, Quievryn G, Messer J, Motylevich Z (2002) Reductive activation with cysteine represents a chromium(III)-dependent pathway in the induction of genotoxicity by carcinogenic chromium(VI). Environ. Health Perspect. 110 (5): 729–731. DOI: 10.1289/ehp.02110s5729.
98. Aiyar J, De Flora S, Wetterhahn KE (1992) Reduction of chromium(vi) to chromium(v) by rat liver cytosolic and microsomal fractions: Is dt-diaphorase involved? Carcinogenesis 13 (7): 1159–1166. DOI: 10.1093/carcin/13.7.1159.
99. Connett PH, Wetterhahn KE (1983) Metabolism of the carcinogen chromate by cellular constituents. Inorg. Elem. Biochem. DOI: 10.1007/bfb0111319.
100. Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V (1999) Reduction of chromium (vi) and its relationship to carcinogenesis. J. Toxicol. Environ. Heal. - Part B Crit. Rev. 2 (1): 87–104. DOI: 10.1080/109374099281241.
101. Wise JTF, Wang L, Xu J, Zhang Z, Shi X (2019) Oxidative stress of Cr(III) and carcinogenesis. In: The Nutritional Biochemistry of Chromium (III). Elsevier B.V.; 2019. p. 323–340. DOI: 10.1016/b978-0-444-64121-2.00010-6.
102. Kanner J, German JB, Kinsella JE (1987) Initiation of lipid peroxidation in biological systems. CRC Crit. Rev. Food Sci. Nutr. 25 (4): 317–364. DOI: 10.1080/10408398709527457.
103. Shi X, Dalal NS (1989) Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI). Biochem. Biophys. Res. Commun. 163 (1): 627–634. DOI: 10.1016/0006-291X(89)92183-9.
104. Ye J, Zhang X, Young HA, Mao Y, Shi X (1995) Chromium(VI)-induced nuclear factor-kB activation in intact cells via free radical reactions. Carcinogenesis 16 (10): 2401–2405. DOI: 10.1093/carcin/16.10.2401.
105. Aiyar J, Berkovits HJ, Floyd RA, Wetterhahn KE (1990) Reaction of chromium(VI) with hydrogen peroxide in the presence of glutathione: reactive intermediates and resulting DNA damage. Chem. Res. Toxicol. 3 (6): 595–603. DOI: 10.1021/tx00018a016.
106. Aiyar J, Borges KM, Floyd RA, Wetterhahn KE (1989) Role of chromium(V), glutathione thiyl radical and hydroxyl radical intermediates in chromium(VI)-induced DNA damage. Toxicol. Environ. Chem. 22 (1–4): 135–148. DOI: 10.1289/ehp.919253.
107. Aiyar J, Berkovits HJ, Floyd RA, Wetterhahn KE (1991) Reaction of chromium(VI) with glutathione or with hydrogen peroxide: identification of reactive intermediates and their role in chromium(VI)-lnduced DNA damage. Environ. Health Perspect. 92: 53–62. DOI: DOI: 10.1289/ehp.919253.
108. Blanchard-Fillion B, Servy C, Ducrocq C (2001) 1-nitrosomelatonin is a spontaneous NO-releasing compound. Free Radic. Res. 35 (6): 857–866. DOI: 10.1080/10715760100301351.
109. Gutteridge JMC HB (1990) The measurement and mechanism of lipid peroxidation in biological systems. Science 15 (4): 129–135. DOI: 10.1016/0968-0004(90)90206-q.
110. Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am. J. Clin. Nutr. 57 (5): 715–725. DOI: 10.1093/ajcn/57.5.715S.
111. Upadhyay RK, Panda SK (2010) Influence of chromium salts on increased lipid peroxidation and differential pattern in antioxidant metabolism in Pistia stratiotes L. Brazilian Arch. Biol. Technol. 53 (5): 1137–1144. DOI: 10.1590/S1516-89132010000500008.
112. Mattagajasingh SN MB and MH (2008) Carcinogenic chromium(VI)-induced protein oxidation and lipid peroxidation: implications in DNA–protein crosslinking. J. Appl. Toxicol. 28: 987–997. DOI: 10.1002/jat.1364.
113. Dey SK, Roy S (2010) Role of GSH in the amelioration of chromium-induced membrane damage. Toxicol. Environ. Chem. 92 (2): 261–269. DOI: 10.1080/02772240902955669.
114. Susa N, Ueno S, Furukawa Y, Michiba N MS (1989) Induction of lipid peroxidation in mice by hexalent chromium and its relation to tthe toxicity. Japanese J. Vet. Sci. 51 (6): 1103–1110. DOI: 10.1292/jvms1939.51.1103.
115. Huang YL, Chen CY, Sheu JY, Chuang IC, Pan JH, Lin TH (1999) Lipid peroxidation in workers exposed to hexavalent chromium. J. Toxicol. Environ. Heal. - Part A. 56 (4): 235–247. DOI: 10.1080/009841099158088.
116. F MA and A (2016) Lipid peroxidation and biochemical abnormalities in tannery workers exposed to hexavalent chromium. Res. J. Biotechnol. 11 (7): 75–82.
117. Ahmad MK, Syma S, Mahmood R (2011) Cr(VI) induces lipid peroxidation, protein oxidation and alters the activities of antioxidant enzymes in human erythrocytes. Biol. Trace Elem. Res. 144 (1–3): 426–435. DOI: 10.1007/s12011-011-9119-5.
118. Kalahasthi RB, Rao RHR, Murthy RBK, Kumar MK (2006) Effect of chromium(VI) on the status of plasma lipid peroxidation and erythrocyte antioxidant enzymes in chromium plating workers. Chem. Biol. Interact. 164 (3): 192–199. DOI: 10.1016/j.cbi.2006.09.012.
119. Asatiani N, Kartvelishvili T, Abuladze M, Asanishvili L, Sapojnikova N (2011) Chromium (VI) can activate and impair antioxidant defense system. Biol. Trace Elem. Res. 142 (3): 388–397. DOI: 10.1007/s12011-010-8806-y.
120. Bagchi D, Vuchetich PJ, Bagchi M, Hassoun EA, Tran MX, Tang L, Stohs SJ (1997) Induction of oxidative stress by chronic administration of sodium dichromate [chromium VI] and cadmium chloride [cadmium II] to rats. Free Radic. Biol. Med. 22 (3): 471–478. DOI: 10.1016/S0891-5849(96)00352-8.
121. Patlolla AK, Barnes C, Yedjou C, Velma VR TP (2006) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley Rats. Environ. Toxicol. 24 (1): 66-73.
122. Sahu BD, Koneru M, Bijargi SR, Kota A, Sistla R (2014) Chromium-induced nephrotoxicity and ameliorative effect of carvedilol in rats: Involvement of oxidative stress, apoptosis and inflammation. Chem. Biol. Interact. 223: 69–79. DOI: 10.1016/j.cbi.2014.09.009.
123. Madejczyk MS, Baer CE, Dennis WE, Minarchick VC, Leonard SS, Jackson DA, Stallings JD, Lewis JA (2015) Temporal changes in rat liver gene expression after acute cadmium and chromium exposure. PLoS One 10 (5): 1–27. DOI: 10.1371/journal.pone.0127327.
124. Mehany HA, Abo-youssef AM, Ahmed LA, Arafa E-SA, Abd El-Latif HA (2013) Protective effect of vitamin E and atorvastatin against potassium dichromate-induced nephrotoxicity in rats. Beni-Suef Univ. J. Basic Appl. Sci. 2 (2): 96–102. DOI: 10.1016/j.bjbas.2013.02.002.
125. Banerjee S, Joshi N, Mukherjee R, Singh PK, Baxi D, Ramachandran A V. (2017) Melatonin protects against chromium (VI) induced hepatic oxidative stress and toxicity: Duration dependent study with realistic dosage. Interdiscip. Toxicol. 10 (1): 20–29. DOI: 10.1515/intox-2017-0003.
126. Han B, Li S, Lv Y, Yang D, Li J, Yang Q, Wu P, Lv Z, Zhang Z (2019) Dietary melatonin attenuates chromium-induced lung injury: Via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 10 (9): 5555–5565.
127. Ren Z, He H, Zuo Z, Xu Z, Wei Z, Deng J (2019) The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. Mol. Biol. Lett. 24:36. doi: 10.1186/s11658-019-0158-9.
128. Chang HC, Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 25 (3): 1–8. DOI: 10.1016/j.tem.2013.12.001.
129. Sathya M, Moorthi P, Premkumar P, Kandasamy M, Jayachandran KS, Anusuyadevi M (2017) Resveratrol intervenes cholesterol-and isoprenoid-mediated amyloidogenic processing of AβPP in familial Alzheimer’s disease. J. Alzheimer’s Dis. 60 (1): S3–S23.
130. Austin S, St-pierre J (2012) PGC1 a and mitochondrial metabolism – emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 125: 4963–4971.
131. Rius-pérez S, Torres-cuevas I, Millán I, Ortega ÁL, Pérez S (2020) PGC-1α , inflammation , and oxidative stress : an integrative view in metabolism. Oxid. Med. Cell. Longev. 2020: 1452696.
132. Awe J, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G (2012) PGC-1 family coactivators and cell fate: Roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signalling. Mitochondrion 12 (1): 86–99. DOI: 10.1016/j.mito.2011.09.009.
133. Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1 (6): 361–370. DOI: 10.1016/j.cmet.2005.05.004.
134. Cantó C, Auwerx J (2009) PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 20 (2): 98–105. DOI: 10.1097/MOL.0b013e328328d0a4.
135. Wang S-J, Zhao X-H, Chen W, Bo N, Wang X-J, Chi Z-F, Wu W (2015) Sirtuin 1 activation enhances the PGC-1α/mitochondrial antioxidant system pathway in status epilepticus. Mol. Med. Rep. 11 (1): 521–526. DOI: 10.3892/mmr.2014.2724.
136. Zheng X, Li S, Li J, Lv Y, Wang X, Wu P, Yang Q, Tang Y, Liu Y, Zhang Z (2020) Hexavalent chromium induces renal apoptosis and autophagy via disordering the balance of mitochondrial dynamics in rats. Ecotoxicol. Environ. Saf. 204: 1–9. DOI: 10.1016/j.ecoenv.2020.111061.
137. Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by Nrf2. Antioxid. Redox. Signal. 29 (17): 1727–1745. DOI: 10.1089/ars.2017.7342.
138. Saha S, Buttari B, Panieri E, Profumo E, Saso L (2020) An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 25 (22): 5474. DOI: 10.3390/molecules25225474.
139. Li L, Dong H, Song E, Xu X, Liu L, Song Y (2013) Nrf2/ARE pathway activation , HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 209: 56-67 DOI: 10.1016/j.cbi.2013.12.005.
140. Ross D, Siegel D (2017) Functions of NQO1 in cellular protection and CoQ10 metabolism and its potential role as a redox sensitive molecular switch. Front. Physiol. 8: 1–10. DOI: 10.3389/fphys.2017.00595.
141. Shen J, Rasmussen M, Dong Q, Tepel M, Scholze A (2017) Expression of the NRF2 target gene NQO1 is enhanced in mononuclear cells in human chronic kidney disease. Oxid. Med. Cell. Longev. 2017: 1–8. DOI: 10.1155/2017/9091879.
142. Torisu-Itakura H, Furue M, Kuwano M, Ono M (2000) Co-expression of thymidine phosphorylase and heme oxygenase-1 in macrophages in human malignant vertical growth melanomas. Jpn J. Cancer Res. 91 (9): 906–910. DOI: 10.1111/j.1349-7006.2000.tb01033.x.
143. Jeong JY, Cha H, Choi EO, Kim CH, Kim G, Yoo YH, Hwang H, Park HT, Yoon HM, Choi YH (2019) Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int. J. Med. Sci. 16 (1): 145–155. DOI: 10.7150/ijms.27005.
144. Zhang X, Ding M, Zhu P, Huang H, Zhuang Q, Shen J, Cai Y, Zhao M, He Q (2019) New insights into the Nrf-2/HO-1 signaling axis and its application in pediatric respiratory diseases. Oxid. Med. Cell. Longev. 2019: 1–9. DOI: 10.1155/2019/3214196.
145. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 73 (17): 3221–3247. DOI: 10.1007/s00018-016-2223-0.
146. Chau L (2015) Heme oxygenase-1: emerging target of cancer therapy. J. Biomed. Sci. 22 (1): 22. DOI: 10.1186/s12929-015-0128-0.
147. MacFie A, Hagan E, Zhitkovich A (2010) Mechanism of DNA-protein cross-linking by chromium. Chem. Res. Toxicol. 23 (2): 341–347. DOI: 10.1021/tx9003402.
148. Das A, Mishra S (2008) Hexavalent chromium (VI) : Environment pollution and health hazard. J. Environ. Res. Dev. 2 (3): 386–392.
149. Zhitkovich A (2005) Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI). Chem. Res. Toxicol. 18 (3): 3–11. DOI: 10.1021/tx049774+.
150. Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 13 (3): 3145–3175. DOI: 10.3390/ijms13033145.
151. Wiegand HJ, Ottenwälder H, Bolt HM 1985. (1985) The Formation of glutathione-chromium complexes and their possible role in chromium disposition. In: Receptors and other targets for toxic substances. 1985. p. 319–321. DOI: 10.1007/978-3-642-69928-3_61.
152. Connett PH, Wetterhahn KE (1985) In vitro reaction of the carcinogen chromate with cellular thiols and carboxylic acids. J. Am. Chem. Soc. 107 (14): 4282–4288. DOI: 10.1021/ja00300a035.
153. Misra HP (1974) Generation of superoxide free radical during the autoxidation of thiols. J. Biol. Chem. 249 (7): 2151–2155.
154. Rowley DA, Halliwell B (1982) Superoxide-dependent formation of hydroxyl radicals in the presence of thiol compounds. FEBS Lett. 138 (1): 33–36. DOI: 10.1016/0014-5793(82)80388-8.
155. O’Brien P, Wang G, Wyatt PB (1992) Studies of the kinetics of the reduction of chromate by glutathione and related thiols. Polyhedron 11 (24): 3211–3216. DOI: 10.1016/S0277-5387(00)83664-5.
156. Kortenkamp A, Oetken G, Beyersmann D (1990) The DNA cleavage induced by a chromium(V) complex and by chromate and glutathione is mediated by activated oxygen species. Mutat. Res. - Fundam. Mol. Mech. Mutagen. 232 (2): 155–161. DOI: 10.1016/0027-5107(90)90120-S.
157. P.faux S, Gao M, Chipman JK, Levy LS (1992) Production of 8-hydroxydeoxyguanosine in isolated DNA by chromium(VI) and chromium(V). Carcinogenesis 13 (9): 1667–1669. DOI: 10.1093/carcin/13.9.1667.
158. Valko M, Izakovic M, Mazur M, Rhodes CJ TJ (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 266 (1–2): 37–56. DOI: 10.1023/b:mcbi.0000049134.69131.89.
159. Tsou T, Chen C, Liu T, Yang J (1996) Induction of 8-hydroxydeoxyguanosine in DNA by chromium(III) plus hydrogen peroxide and its prevention by scavengers. Carcinogenesis 17 (1): 103–108. DOI: 10.1093/carcin/17.1.103.
160. Kuo HW, Chang SF, Wu KY, Wu FY (2003) Chromium (VI) induced oxidative damage to DNA: Increase of urinary 8-hydroxydeoxyguanosine concentrations (8-OHdG) among electroplating workers. Occup. Environ. Med. 60 (8): 590–594. DOI: 10.1136/oem.60.8.590.
161. Zhang XH, Zhang X, Wang XC, Jin LF, Yang ZP, Jiang CX, Chen Q, Ren X Bin, Cao JZ, Wang Q, Zhu YM (2011) Chronic occupational exposure to hexavalent chromium causes DNA damage in electroplating workers. BMC Public Health. 11 (224): 1–8. DOI: 10.1186/1471-2458-11-224.
162. Pan CH, Jeng HA, Lai CH (2018) Biomarkers of oxidative stress in electroplating workers exposed to hexavalent chromium. J. Expo. Sci. Environ. Epidemiol. 28 (1): 76–83. DOI: 10.1038/jes.2016.85.
163. Li P, Gu Y, Yu S, Li Y, Yang J, Jia G (2014) Assessing the suitability of 8-OHdG and micronuclei as genotoxic biomarkers in chromate-exposed workers: A cross-sectional study. BMJ Open 4 (10): 1–7. DOI: 10.1136/bmjopen-2014-005979.
164. Setyaningsih Y, Husodo AH, Astuti I (2015) Detection of urinary 8-hydroxydeoxyguanosine (8-OHdG) levels as a biomarker of oxidative DNA damage among home industry workers exposed to chromium. Procedia Environ. Sci. 23: 290–296. DOI: 10.1016/j.proenv.2015.01.043.
165. Zhitkovich A (2001) Non-oxidative mechanisms are responsible for the induction of mutagenesis by reduction of Cr(VI) with cysteine: Role of ternary DNA adducts in Cr(III)-dependent mutagenesis. Biochemistry 40 (2): 549–560. DOI: 10.1021/bi0015459.
166. Ning J, Grant MH (2000) The role of reduced glutathione and glutathione reductase in the cytotoxicity of chromium (VI) in osteoblasts. Toxicol. Vitr. 14 (4): 329–335. DOI: 10.1016/S0887-2333(00)00024-2.
167. Lalaouni A, Henderson C, Kupper C, Grant MH (2007) The interaction of chromium (VI) with macrophages: Depletion of glutathione and inhibition of glutathione reductase. Toxicology 236 (1–2): 76–81. DOI: 10.1016/j.tox.2007.04.002.
168. Wiegand HJ, Ottenwälder H, Bolt HM (1984) The reduction of chromium (VI) to chromium (III) by glutathione: An intracellular redox pathway in the metabolism of the carcinogen chromate. Toxicology 33 (3–4): 341–348. DOI: 10.1016/0300-483X(84)90050-7.
169. Lu SC (2013) Glutathione synthesis. Biochim. Biophys. Acta. 1830 (5): 3143–3153. DOI: 10.1016/j.bbagen.2012.09.008.
170. Standeven AM, Wetterhahn KE (1991) Possible role of glutathione in chromium(VI) metabolism and toxicity in rats. Pharmacol. Toxicol. 68 (6): 469–476. DOI: 10.1111/j.1600-0773.1991.tb01272.x.
171. Na KJ, Jeong SY, Lim CH (1992) The role of glutathione in the acute nephrotoxicity of sodium dichromate. Arch. Toxicol. 66 (9): 646–651. DOI: 10.1007/BF01981504.
172. Cupo DY, Wetterhahn KE (1985) Modification of chromium(VI)-induced DNA damage by glutathione and cytochromes P-450 in chicken embryo hepatocytes. Proc. Natl. Acad. Sci. U. S. A. 82 (20): 6755–6759. DOI: 10.1073/pnas.82.20.6755.
173. Standeven AM, Wetterhahn KE (1991) Ascorbate is the principal reductant of chromium(VI) in rat liver and kidney ultrafiltrates. Carcinogenesis 12 (9): 1733–1737. DOI: 10.1093/carcin/12.9.1733.
174. Gunaratnam M, Grant MH (2001) The role of glutathione reductase in the cytotoxicity of chromium (VI) in isolated rat hepatocytes. Chem. Biol. Interact. 134 (2): 191–202. DOI: 10.1016/S0009-2797(01)00153-3.
175. Suzuki Y, Fukuda K (1990) Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch. Toxicol. 64 (3): 169–176. DOI: 10.1007/BF02010721.
176. Standeven AM, Wetterhahn KE, Kato R (1992) Ascorbate is the principal reductant of chromium(vi) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis 13 (8): 1319–1324. DOI: 10.1093/carcin/13.8.1319.
177. Stearns DM, Wetterhahn KE (1994) Reaction of chromium(VI) with ascorbate produces chromium(V), chromium(IV), and carbon-based radicals. Chem. Res. Toxicol. 7 (2): 219–230. DOI: 10.1021/tx00038a016.
178. Stearns DM, Courtney KD, Giangrande PH, Phieffer LS, Wetterhahn KE (1994) Chromium(VI) reduction by ascorbate: Role of reactive intermediates in DNA damage in vitro. Environ. Health Perspect. 102 (3): 21–25. DOI: 10.1289/ehp.94102s321.
179. Goodgame DML, Joy AM (1987) EPR study of the Cr(V) and radical species produced in the reduction of Cr(VI) by ascorbate. Inorganica Chim. Acta. 135 (2): 115–118. DOI: 10.1016/S0020-1693(00)83273-9.
180. Steams DM, Kennedy LJ, Courtney KD, Giangrande PH, Phieffer LS, Wetterhahn KE (1995) Reduction of chromium(VI) by ascorbate leads to chromium-dna binding and DNA strand breaks in vitro. Biochemistry 34 (3): 910–919. DOI: 10.1021/bi00003a025.
181. Bielski BHJ, Allen AO, Schwarz HA (1981) Mechanism of disproportionation of ascorbate radicals. J. Am. Chem. Soc. 103 (12): 3516–3518. DOI: 10.1021/ja00402a042.
182. Sugiyama M, Ando A, Ogura R (1989) Effect of vitamin E on survival, glutathione reductase and formation of chromium (V) in Chinese hamster V-79 cells treated with sodium chromate (VI). Carcinogenesis 10 (4): 737–741. DOI: 10.1093/carcin/10.4.737.
183. Poljsak B, Gazdag Z, Jenko-Brinovec S, Fujs S, Pesti M, Belagyi J, Plesničar S, Raspor P (2005) Pro-oxidative vs antioxidative properties of ascorbic acid in chromium(VI)-induced damage: An in vivo and in vitro approach. J. Appl. Toxicol. 25 (6): 535–548. DOI: 10.1002/jat.1093.
184. Quievryn G, Messer J, Zhitkovich A (2006) Lower mutagenicity but higher stability of Cr-DNA adducts formed during gradual chromate activation with ascorbate. Carcinogenesis 27 (11): 2316–2321. DOI: 10.1093/carcin/bgl076.
185. Quievryn G, Peterson E, Messer J, Zhitkovich A (2003) Genotoxicity and mutagenicity of chromium(VI)/ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry. 42 (4): 1062–1070. DOI: 10.1021/bi0271547.
186. Kortenkamp A, O’Brien P (1994) The generation of DNA single-strand breaks during the reduction of chromate by ascorbic acid and/or glutathione in vitro. Environ. Health Perspect. 102 (SUPPL 3): 237–241. DOI: 10.1289/ehp.94102s3237.
187. Flora SD BB and LA (1984) Distinctive mechanisms for interaction of hexavalent and trivalent chromium with DNA? Toxicol. Environ. Chem. 8 (4): 287–294. DOI: 10.1080/02772248409357060.
188. Sugiyama M (1992) Role of physiological antioxidants in chromium (VI)- induced cellular injury. Free Radic. Biol. Med. 12 (5): 397–407. DOI: 10.1016/0891-5849(92)90089-y.
189. Tsapakos MJ, Wetterhahn KE (1983) The interaction of chromium with nucleic acids. Chem. Biol. Interact. 46 (2): 265–277. DOI: 10.1016/0009-2797(83)90034-0.
190. Arakawa H, Weng MW, Chen WC, Tang MS (2012) Chromium (VI) induces both bulky DNA adducts and oxidative DNA damage at adenines and guanines in the p53 gene of human lung cells. Carcinogenesis 33 (10): 1993–2000. DOI: 10.1093/carcin/bgs237.
191. Rossi SC, Wetterhahn KE (1989) Chromium (V) is produced upon reduction of chromate by mitochondrial electron transport chain complexes. Carcinogenesis 10 (5): 913–930. DOI: 10.1093/carcin/10.5.913.
192. Myers CR (2012) The effects of chromium(VI) on the thioredoxin system: Implications for redox regulation. Free Radic. Biol. Med. 52 (10): 2091–2107. DOI: 10.1016/j.freeradbiomed.2012.03.013.
193. Arillo A, Melodia F, Frache R (1987) Reduction of hexavalent chromium by mitochondria: Methodological implications and possible mechanisms. Ecotoxicol. Environ. Saf. 14 (2): 164–177. DOI: 10.1016/0147-6513(87)90059-5.
194. Myers JM, Antholine WE, Myers CR (2011) The intracellular redox stress caused by hexavalent chromium is selective for proteins that have key roles in cell survival and thiol redox control. Toxicology 281 (1–3): 37–47. DOI: 10.1016/j.tox.2011.01.001.
195. Myers JM, Myers CR (2009) The effects of hexavalent chromium on thioredoxin reductase and peroxiredoxins in human bronchial epithelial cells. Free Radic. Biol. Med. 47 (10): 1477–1485. DOI: 10.1016/j.freeradbiomed.2009.08.015.
196. Bhattacharya M, Shriwastav A, Bhole S, Silori R, Mansfeldt T, Kretzschmar R, Singh A (2020) Processes governing chromium contamination of groundwater and soil from a chromium waste source. ACS Earth Sp. Chem. 4 (1): 35–49. DOI: Https://doi.org/10.1021/acsearthspacechem.9b00223.
197. Hald M, Agner T, Blands J, Ravn H, Johansen JD (2009) Allergens associated with severe symptoms of hand eczema and a poor prognosis. Contact Dermatitis 61 (2): 101–108.
198. Hansen MB, Menné T, Johansen JD (2006) Cr(III) reactivity and foot dermatitis in Cr(VI) positive patients. Contact Dermatitis 54 (3): 140–144. DOI: 10.1111/j.0105-1873.2006.00802.x.
199. Wang BJ, Wu J De, Sheu SC, Shih TS, Chang HY, Guo YL, Wang YJ, Chou TC (2011) Occupational hand dermatitis among cement workers in Taiwan. J. Formos. Med. Assoc. 110 (12): 775–779. DOI: 10.1016/j.jfma.2011.11.008.
200. Thyssen JP, Jensen P, Carlsen BC, Engkilde K, Menné T, Johansen JD (2009) The prevalence of chromium allergy in Denmark is currently increasing as a result of leather exposure. Br. J. Dermatol. 161 (6): 1288–1293. DOI: 10.1111/j.1365-2133.2009.09405.x.
201. Caroe C, Andersen KE, Thyssen JP, Mortz CG (2010) Fluctuations in the prevalence of chromate allergy in Denmark and exposure to chrome-tanned leather. Contact Dermatitis 63 (6): 340–346. DOI: 10.1111/j.1600-0536.2010.01798.x.
202. Thyssen JP, Menné T (2010) Metal allergys-A review on exposures, penetration, genetics, prevalence, and clinical implications. Chem. Res. Toxicol. 23 (2): 309–318. DOI: 10.1021/tx9002726.
203. Stocks SJ, McNamee R, Turner S, Carder M, Agius RM (2012) Has European Union legislation to reduce exposure to chromate in cement been effective in reducing the incidence of allergic contact dermatitis attributed to chromate in the UK? Occup. Environ. Med. 69 (2): 150–152. DOI: 10.1136/oemed-2011-100220.
204. Hansen MB, Johansen JD, Menné T (2003) Chromium allergy: Significance of both Cr(III) and Cr(VI). Contact Dermatitis 49 (4): 206–212. DOI: 10.1111/j.0105-1873.2003.0230.x.
205. Hedberg YS, Lidén C, Odnevall Wallinder I (2015) Chromium released from leather - I: Exposure conditions that govern the release of chromium(III) and chromium(VI). Contact Dermatitis 72 (4): 206–215. DOI: 10.1111/cod.12329.
206. Sharma P, Bihari V, Agarwal SK, Verma V, Kesavachandran CN, Pangtey BS, Mathur N, Singh KP, Srivastava M, Goel SK (2012) Groundwater contaminated with hexavalent chromium [Cr(VI)]: A health survey and clinical examination of community inhabitants (Kanpur, India). PLoS One 7 (10): 3–9. DOI: 10.1371/journal.pone.0047877.
207. Singhal VK, Deswal BS, Singh BN (2015) Study of skin and mucous membrane disorders among workers engaged in the sodium dichromate manufacturing industry and chrome plating industry. Indian J. Occup. Environ. Med. 19 (3): 129–133. DOI: 10.4103/0019-5278.173994.
208. Wedeen RP, Qian LF (1991) Chromium-induced kidney disease. Environ. Health Perspect 92 : 71–74. DOI: 10.1289/ehp.92-1519395.
209. Pieri C, Moroni F, Marra M, Marcheselli F, Recchioni R (1995) Melatonin is an efficient antioxidant. Arch. Gerontol. Geriatr. 20 (2): 159–165. DOI: 10.1016/0167-4943(94)00593-V.
210. Poeggeler B, Reiter RJ, Hardeland R, Tan DX, Barlow-Walden LR (1996) Melatonin and structurally-related, endogenous indoles act as potent electron donors and radical scavengers in vitro. Redox Rep. 2 (3): 179–184. DOI: 10.1080/13510002.1996.11747046.
211. Tan D, Reiter R, Manchester L, Yan M, El-Sawi M, Sainz R, Mayo J, Kohen R, Allegra M, Hardelan R (2005) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2 (2): 181–197. DOI: 10.2174/1568026023394443.
212. Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow‐Walden L, Chuang J, Ortiz GG, AcuñaCastroviejo D (1995) A review of the evidence supporting melatonin’s role as an antioxidant. J. Pineal Res. 18 (1): 1–11. DOI: 10.1111/j.1600-079X.1995.tb00133.x.
213. Sofic E, Rimpapa Z, Kundurovic Z, Sapcanin A, Tahirovic I, Rustembegovic A, Cao G (2005) Antioxidant capacity of the neurohormone melatonin. J. Neural Transm. 112 (3): 349–358. DOI: 10.1007/s00702-004-0270-4.
214. Loren P, Sánchez R, Arias ME, Felmer R, Risopatrón J, Cheuquemán C (2017) Melatonin scavenger properties against oxidative and nitrosative stress: Impact on gamete handling and in vitro embryo production in humans and other mammals. Int. J. Mol. Sci. 18 (6): 1–17. DOI: 10.3390/ijms18061119.
215. Reiter RJ, Tan DX, Manchester LC, Lopez-Burillo S, Sainz RM, Mayo JC (2003) Melatonin: Detoxification of oxygen and nitrogen-based toxic reactants. Adv. Exp. Med. Biol. 527: 539–548. DOI: 10.1007/978-1-4615-0135-0_62.
216. Arnao MB, Hernández-Ruiz J (2019) Melatonin and reactive oxygen and nitrogen species: a model for the plant redox network. Melatonin Res. 2 (3): 152–168. DOI: 10.32794/11250036.
217. Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC (2007) Melatonin role in the mitochondrial function. Front. Biosci. 12 (3): 947–963. DOI: 10.2741/2116.
218. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 51 (1): 1–16. DOI: 10.1111/j.1600-079X.2011.00916.x.
219. Chan TY, Tang PL (1996) Characterization of the antioxidant effects of melatonin and related indoleamines in vitro. J. Pineal Res. 20 (4): 187–191. DOI: 10.1111/j.1600-079X.1996.tb00257.x.
220. Cagnoli CM, Atabay C, Kharlamova E, Manev H (1995) Melatonin protects neurons from singlet oxygen‐induced apoptosis. J. Pineal Res. 18 (4): 222–226. DOI: 10.1111/j.1600-079X.1995.tb00163.x.
221. Matuszak Z, Bilska MA, Reszka KJ, Chignell CF, Bilski P (2003) Interaction of singlet molecular oxygen with melatonin and related indoles. Photochem. Photobiol. 78 (5): 449. DOI: 10.1562/0031-8655(2003)078<0449:iosmow>2.0.co;2.
222. Schaefer M, Hardeland R (2009) The melatonin metabolite N1-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J. Pineal Res. 46 (1): 49–52. DOI: 10.1111/j.1600-079X.2008.00614.x.
223. Zang LY, Cosma G, Gardner H, Vallyathan V (1998) Scavenging of reactive oxygen species by melatonin. Biochim. Biophys. Acta - Gen. Subj. 1425 (3): 469–477. DOI: 10.1016/S0304-4165(98)00099-3.
224. Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ (2003) Oxidative damage to catalase induced by peroxyl radicals: Functional protection by melatonin and other antioxidants. Free Radic. Res. 37 (5): 543–553. DOI: 10.1080/1071576031000083206.
225. Longoni B, Salgo MG, Pryor WA, Marchiafava PL (1998) Effects of melatonin on lipid peroxidation induced by oxygen radicals. Life Sci. 62 (10): 853–859. DOI: 10.1016/S0024-3205(98)00002-2.
226. Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F (1994) Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 55 (15): 271–276. DOI: 10.1016/0024-3205(94)00666-0.
227. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B (1996) Evaluation of the antioxidant activity of melatonin in vitro. Free Radic. Biol. Med. 21 (3): 307–315. DOI: 10.1016/0891-5849(96)00046-9.
228. Matuszak Z, Reszka KJ, Chignell CF (1997) Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic. Biol. Med. 23 (3): 367–372. DOI: 10.1016/S0891-5849(96)00614-4.
229. Stasica P, Paneth P, Rosiak JM (2000) Hydroxyl radical reaction with melatonin molecule: A computational study. J. Pineal Res. 29 (2): 125–127. DOI: 10.1034/j.1600-079X.2000.290209.x.
230. Tan DX, Chen LD, Poeggeler B, Manchester LC and RR (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1 : 57–60.
231. Ceraulo L, Ferrugia M, Tesoriere L, Segreto S, Livrea MA, Turco Liveri V (1999) Interactions of melatonin with membrane models: Portioning of melatonin in AOT and lecithin reversed micelles. J. Pineal Res. 26 (2): 108–112. DOI: 10.1111/j.1600-079X.1999.tb00570.x.
232. Kaya H, Delibas N, Serteser M, Ulukaya E, Özkaya O (1999) The effect of melatonin on lipid peroxidation during radiotherapy in female rats. Strahlentherapie und Onkol. 175 (6): 285–288. DOI: 10.1007/BF02743581.
233. Taysi S, Koc M, Büyükokuroǧlu ME, Altinkaynak K, Şahin YN (2003) Melatonin reduces lipid peroxidation and nitric oxide during irradiation-induced oxidative injury in the rat liver. J. Pineal Res. 34 (3): 173–177. DOI: 10.1034/j.1600-079X.2003.00024.x.
234. Daniels WMU, Van Rensburg SJ, Van Zyl JM, Taljaard JJF (1998) Melatonin prevents β-amyloid-induced lipid peroxidation. J. Pineal Res. 24 (2): 78–82. DOI: 10.1111/j.1600-079X.1998.tb00370.x.
235. Karbownik M, Lewiński A (2003) Melatonin reduces fenton reaction-induced lipid peroxidation in porcine thyroid tissue. J. Cell. Biochem. 90 (4): 806–811. DOI: 10.1002/jcb.10689.
236. García JJ, Reiter RJ, Guerrero JM, Escames G, Yu BP, Oh CS, Munoz-Hoyos A (1997) Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 408 (3): 297–300. DOI: 10.1016/S0014-5793(97)00447-X.
237. García JJ, Reiter RJ, Ortiz GG, Oh CS, Tang L, Yu BP, Escames G (1998) Melatonin enhances tamoxifen’s ability to prevent the reduction in microsomal membrane fluidity induced by lipid peroxidation. J. Membr. Biol. 162 (1): 59–65. DOI: 10.1007/s002329900342.
238. Livrea MA, Tesoriere L, Arpa D, Morreale M (1997) Reaction of melatonin with lipoperoxyl radicals in phospholipid bilayers. Free Radic. Biol. Med. 23 (5): 706–711. DOI: 10.1016/S0891-5849(97)00018-X.
239. Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S (2001) Free radical-mediated molecular damage: Mechanisms for the protective actions of melatonin in the central nervous system. Ann. N. Y. Acad. Sci. 939: 200–215. DOI: 10.1111/j.1749-6632.2001.tb03627.x.
240. Manda K, Ueno M, Anzai K (2007) AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J. Pineal Res. 42 (4): 386–393. DOI: 10.1111/j.1600-079X.2007.00432.x.
241. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36 (1): 1–9. DOI: 10.1046/j.1600-079X.2003.00092.x.
242. Mayo JC, Sainz RM, Antolín I, Herrera F, Martin V, Rodriguez C (2002) Melatonin regulation of antioxidant enzyme gene expression. Cell. Mol. Life Sci. 59 (10): 1706–1713. DOI: 10.1007/PL00012498.
243. Reiter RJ, Tan DX, Carmen O EG (2000) Actions of melatonin in the reduction of oxidative stress. J. Biomed. Sci. 7 (6): 444–458. DOI: 10.1007/BF02253360.
244. Acuña-Castroviejo D, Escames G, León J, Carazo A, Khaldy H (2003) Mitochondrial regulation by melatonin and its metabolites. Adv. Exp. Med. Biol. 527: 549–557. DOI: 10.1007/978-1-4615-0135-0_63.
245. Siu AW, Ortiz GG, Benitez-King G, To CH, Reiter RJ (2004) Effect of melatonin on the nitric oxide treated retina. Br. J. Ophthalmol. 88 (8): 1078–1081. DOI: 10.1136/bjo.2003.037879.
246. Tapias V, Escames G, López LC, López A, Camacho E, Carrión MD, Entrena A, Gallo MA, Espinosa A, Acuña-Castroviejo D (2009) Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in Parkinsonian mice. J. Neurosci. Res. 87 (13): 3002–3010. DOI: 10.1002/jnr.22123.
247. Zhang H, Squadrito GL, Uppu R, Pryor WA (1999) Reaction of peroxynitrite with melatonin: A mechanistic study. Chem. Res. Toxicol. 12 (6): 526–534. DOI: 10.1021/tx980243t.
248. Pozo D, Reiter RJ, Calvo JR, Guerrero JM (1994) Physiological concentrations of melatonin inhibit nitric oxide synthase in rat cerebellum. Life Sci. 55 (24): 455–460. DOI: 10.1016/0024-3205(94)00532-X.
249. Guo P, Pi H, Xu S, Zhang L, Li Y, Li M, Cao Z, Tian L, Xie J (2014) Melatonin improves mitochondrial function by promoting cadmium-induced hepatotoxicity in vitro. Toxicol. Sci. 142 (1): 182–195.
250. Naaz S, Mishra S, Pal PK, Chattopadhyay A, Das AR, Bandyopadhyay D (2020) Activation of SIRT1/PGC 1α/SIRT3 pathway by melatonin provides protection against mitochondrial dysfunction in isoproterenol induced myocardial injury. Heliyon 6 (10). DOI: 10.1016/j.heliyon.2020.e05159.
251. Kumar J, Haldar C, Verma R (2020) Fluoride compromises testicular redox sensor, gap junction protein, and metabolic status: amelioration by melatonin. Biol. Trace Elem. Res. 196 (2): 552–564. DOI: 10.1007/s12011-019-01946-6.
252. Kleszczyński K, Zillikens D, Fischer TW (2016) Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet rad. J. Pineal Res. 61 (2): 187–197. DOI: 10.1111/jpi.12338.
253. Kumar J, Verma R, Haldar C (2021) Melatonin ameliorates bisphenol S induced testicular damages by modulating Nrf-2/HO-1 and SIRT-1/FOXO-1 expressions. Environ. Toxicol. 36 (3): 396–407. DOI: 10.1002/tox.23045.
254. Mitra E, Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Res. 2 (2): 1–21. DOI: 10.32794/mr11250018.
255. Urata Y, Honma S, Goto S, Todoroki S, Iida T, Cho S, Honma K, Kondo T (1999) Melatonin induces γ-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 27 (7–8): 838–847. DOI: 10.1016/S0891-5849(99)00131-8.
256. Ionescu JG, Poljsak B (2011) Metal ions mediated pro-oxidative reactions with vitamin c: Possible implications for treatment of different malignancies. Cancer Prev. Res. Perspect 3 (3): 149–182.
257. Porter WL (1993) Paradoxical behavior of antioxidants in food and biological systems. Toxicol. Ind. Health. 9 (1–2): 93–122. DOI: 10.1177/0748233793009001-209.
258. Guajardo MH, Terrasa AM, Catalá A (2006) Lipid-protein modifications during ascorbate-Fe2+ peroxidation of photoreceptor membranes: Protective effect of melatonin. J. Pineal Res. 41 (3): 201–210. DOI: 10.1111/j.1600-079X.2006.00352.x.
259. Du J, Wagner BA, Buettner GR, Cullen JJ (2015) Role of labile iron in the toxicity of pharmacological ascorbate. Free Radic. Biol. Med. 84: 289–295. DOI: 10.1016/j.freeradbiomed.2015.03.033.
260. Ghosh AK, Naaz S, Bhattacharjee B, Ghosal N, Chattopadhyay A, Roy S, Reiter RJ, Bandyopadhyay D (2017) Mechanism of melatonin protection against copper-ascorbate-induced oxidative damage in vitro through isothermal titration calorimetry. Life Sci. 180: 123–136. DOI: 10.1016/j.lfs.2017.05.022.
261. Kobayashi S UK, Morita J SH, T K (1988) DNA damage induced by ascorbate in the presence of Cu2+. Biochim. Biophys. Acta - Gene Struct. Expr. 949 (1): 143–147. DOI: 10.1016/0167-4781(88)90065-6.
262. Qi W, Reiter RJ, Tan DX, Manchester LC, Siu AW, Garcia JJ (2000) Increased levels of oxidatively damaged DNA induced by chromium(III) and H2O2: Protection by melatonin and related molecules. J. Pineal Res. 29 (1): 54–61. DOI: 10.1034/j.1600-079X.2000.290108.x.
263. Hneihen AS, Standeven AM, Wetterhahn KE (1993) Differential binding of chromium(VI) and chromium(III) complexes to salmon sperm nuclei and nuclear DNA and isolated calf thymus DNA. Carcinogenesis 14 (9): 1795–1803. DOI: 10.1093/carcin/14.9.1795.
264. Susa N, Ueno S, Furukawa Y, Ueda J, Sugiyama M (1997) Potent protective effect of melatonin on chromium(VI)-induced DNA single-strand breaks, cytotoxicity, and lipid peroxidation in primary cultures of rat hepatocytes. Toxicol. Appl. Pharmacol. 144 (2): 377–384. DOI: 10.1006/taap.1997.8151.
265. Acuña-Castroviejo D, Martín M, Macías M, Escames G, León J, Khaldy H, Reiter RJ (2001) Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 30 (2): 65–74. DOI: 10.1034/j.1600-079X.2001.300201.x.
266. Menendez‐Pelaez A, Reiter RJ (1993) Distribution of melatonin in mammalian tissues: The relative importance of nuclear versus cytosolic localization. J. Pineal Res. 15 (2): 59–69. DOI: 10.1111/j.1600-079X.1993.tb00511.x.
267. Menendez‐Pelaez A, Poeggeler B, Reiter RJ, Barlow‐Walden L, Pablos MI, Tan DX (1993) Nuclear localization of melatonin in different mammalian tissues: Immunocytochemical and radioimmunoassay evidence. J. Cell. Biochem. 53 (4): 373–382. DOI: 10.1002/jcb.240530415.
268. Peter Bozner, Valentina Grishko, Susan P. LeDoux, Glenn L. Wilson Y-CC and MAP (1997) The amyloid β protein induces oxidative damage of mitochondrial DNA. J. Neuropathol. Exp. Neurol. 56 (12): 1356–1362. DOI: Doi: 10.1097/00005072-199712000-00010.
269. León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ (2005) Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 38 (1): 1–9. DOI: 10.1111/j.1600-079X.2004.00181.x.
270. Leon J, Acuña-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ (2004) Melatonin and mitochondrial function. Life Sci. 75 (7): 765–790. DOI: 10.1016/j.lfs.2004.03.003.
271. Reiter RJ, Paredes SD, Korkmaz A, Tan DX, Jou MJ (2008) Melatonin combats molecular terrorism at the mitochondrial level. Interdiscip. Toxicol. 1 (2): 137–149.
272. Li J, Zheng X, Ma X, Xu X, Du Y, Lv Q, Li X, Wu Y, Sun H, Yu L, Zhang Z (2019) Melatonin protects against chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2 pathway. J. Inorg. Biochem. 97: 110698. doi: 10.1016/j.jinorgbio.2019.110698.
273. Shagirtha K, Muthumani M, Milton Prabu S (2011) Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur. Rev. Med. Pharmacol. Sci. 15 (9): 1039–1050.
274. El-Sokkary GH, Nafady AA, Shabash EH (2010) Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol. Environ. Saf. 73 (3): 456–463. DOI: 10.1016/j.ecoenv.2009.09.014.
275. Yang Q, Zhu J, Luo X, Li F, Cong L, Wang Y, Sun Y (2019) Melatonin attenuates cadmium-induced ovulatory dysfunction by suppressing endoplasmic reticulum stress and cell apoptosis. Reprod. Biol. Endocrinol. 17 (1): 61. DOI: 10.1186/s12958-019-0502-y.
276. Othman AI, El-Missiry MA, Amer MA, Arafa M (2008) Melatonin controls oxidative stress and modulates iron, ferritin, and transferrin levels in adriamycin treated rats. Life Sci. 83 (15–16): 563–568. DOI: 10.1016/j.lfs.2008.08.004.
277. Abd Elkader MAE, Aly HF (2015) Protective effect of melatonin against iron overload-induced toxicity in rats. Int. J. Pharm. Pharm. Sci. 7 (9): 116–121.
278. Parmar P, Limson J, Nyokong T, Daya S (2002) Melatonin protects against copper-mediated free radical damage. J. Pineal Res. 32 (4): 237–242. DOI: 10.1034/j.1600-079X.2002.01859.x.
279. Şener G, Şehirli AO, Ayanoglu-Dülger G (2003) Melatonin protects against mercury(II)-induced oxidative tissue damage in rats. Pharmacol. Toxicol. 93 (6): 290–296. DOI: 10.1111/j.1600-0773.2003.pto930607.x.
280. Rao MV, Chhunchha B (2010) Protective role of melatonin against the mercury induced oxidative stress in the rat thyroid. Food Chem. Toxicol. 48 (1): 7–10. DOI: 10.1016/j.fct.2009.06.038.
281. Rao MV, Gangadharan B (2008) Antioxidative potential of melatonin against mercury induced intoxication in spermatozoa in vitro. Toxicol. Vitr. 22 (4): 935–942.
282. Jindal M, Garg GR, Mediratta PK, Fahim M (2011) Protective role of melatonin in myocardial oxidative damage induced by mercury in murine model. Hum. Exp. Toxicol. 30 (10): 1489–1500.
283. Tapan AP, Mandava VR (2016) Melatonin modulates mercury induced oxidative stress in rat liver. World J. Pharm. Res. 5 (2): 1198–1211.
284. Rao MV, Purohit AR (2011) Neuroprotection by melatonin on mercury induced toxicity in the rat brain. Pharmacol. Amp. Pharm. 02 (04): 375–385.
285. Uygur R, Aktas C, Caglar V, Uygur E, Erdogan H, Ozen OA (2016) Protective effects of melatonin against arsenic-induced apoptosis and oxidative stress in rat testes. Toxicol. Ind. Health 32 (5): 848–859.
286. Dutta S, Saha S, Mahalanobish S, Sadhukhan P, Sil PC (2018) Melatonin attenuates arsenic induced nephropathy via the regulation of oxidative stress and inflammatory signaling cascades in mice. Food Chem. Toxicol. 118: 303-316. doi: 10.1016/j.fct.2018.05.032
287. Singh SS, Deb A, Sutradhar S (2019) Effect of melatonin on arsenic-induced oxidative stress and expression of MT1 and MT2 receptors in the kidney of laboratory mice. Biol. Rhythm Res. 00 (00): 1–15. DOI: 10.1080/09291016.2019.1566993.
288. Durappanavar PN, Nadoor P, Waghe P, Pavithra BH, Jayaramu GM (2019) Melatonin ameliorates neuropharmacological and neurobiochemical alterations induced by subchronic exposure to arsenic in Wistar Rats. Biol. Trace Elem. Res. 190 (1): 124–139. DOI: 10.1007/s12011-018-1537-1.
289. Pal S, Chatterjee AK (2006) Possible beneficial effects of melatonin supplementation on arsenic-induced oxidative stress in Wistar rats. Drug Chem. Toxicol. 29 (4): 423–433.
290. Pal S, Chatterjee AK (2005) Prospective protective role of melatonin against arsenic-induced metabolic toxicity in Wistar rats. Toxicology 208 (1): 25–33.
291. Hernández-Plata E, Quiroz-Compeán F, Ramírez-Garcia G, Barrientos EY, Rodríguez-Morales NM, Flores A, Wrobel K, Wrobel K, Méndez I, Díaz-Muñoz M, Robles J, Martínez-Alfaro M (2015) Melatonin reduces lead levels in blood, brain and bone and increases lead excretion in rats subjected to subacute lead treatment. Toxicol. Lett. 233 (2): 78–83. DOI: 10.1016/j.toxlet.2015.01.009.
292. Bandyopadhyay D (2014) Melatonin protects against lead-induced oxidative stress in stomach, duodenum and spleen of male Wistar rats. J. Pharmacy Res. 1 (11): 997–1004.
293. Al-Olayan EM, El-Khadragy MF, Abdel Moneim AE (2015) The protective properties of melatonin against aluminium-induced neuronal injury. Int. J. Exp. Pathol. 96 (3): 196–202. DOI: 10.1111/iep.12122.
294. Allagui MS, Feriani A, Saoudi M, Badraoui R, Bouoni Z, Nciri R, Murat JC, Elfeki A (2014) Effects of melatonin on aluminium-induced neurobehavioral and neurochemical changes in aging rats. Food Chem. Toxicol. 70 : 84

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


