Biorhythmic and receptor mediated interplay between melatonin and insulin: its consequences on diabetic erythrocytes
Melatonin: a grace for diabetics
Abstract
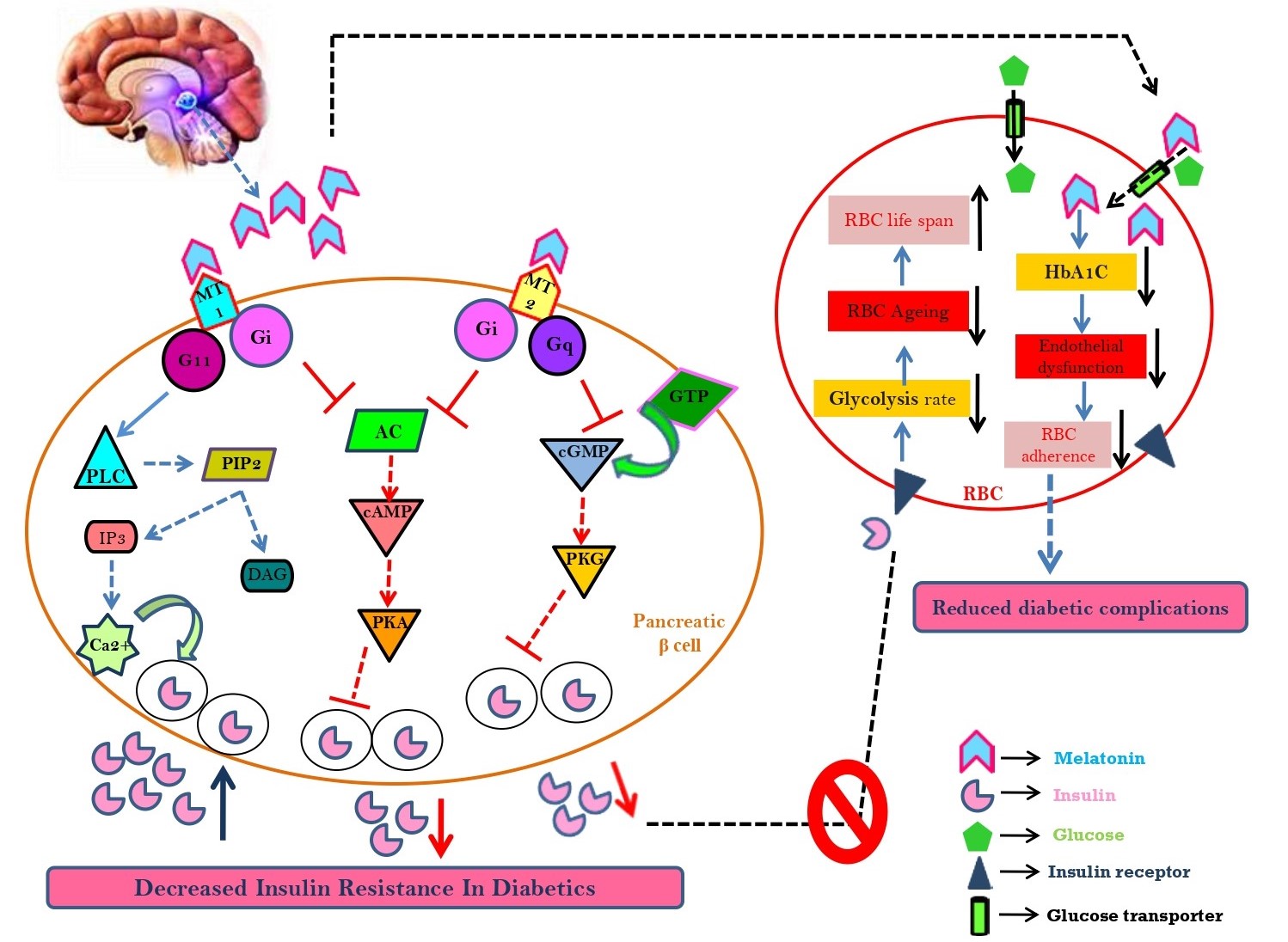
Diabetes mellitus, one of the crucial epidemics of this country has snatched the sleep of mankind with a steep slope of 108 million in 1980 to more than 460 million in today’s world. The global statistics based on numerological information from World Health Organization (WHO) proposed alarmingly about 642 million affected individuals by 2040. Type 1 diabetes is due to damaged pancreatic β-cells while type 2 diabetes is a result of insulin insensitivity associated with hyperglycaemia. Hyperglycaemia is a principal symptom of diabetes. As a result, the circulatory erythrocytes [red blood cells (RBCs)] become the first and most vulnerable victims to confront such a stressful environment. The RBCs possess many components including haemoglobin, membrane proteins and lipids. They prefer to interact with glucose and form glycated haemoglobin and membrane phospholipid asymmetry which alters RBC adherence. These alterations trigger intracellular reactive oxygen species (ROS) formation and oxidative damage in diabetic erythrocytes. Melatonin, an indoleamine, ameliorates oxidative stress in various tissues and has the capacity of shielding erythrocytes from deleterious stress. A crucial relationship between melatonin and insulin indicates their interplay in occurrence of diabetes. Biorhythm entrained and receptor mediated action of melatonin on pancreatic β-cells in the context of hyperglycaemia are discussed for the first time in the review. Since melatonin protects against erythrocytes, as well as beneficial to diabetes, it is worthy to address proficiency of this indoleamine to the diabetic erythrocytes. In summary, this review has discussed the fostering role of melatonin in hyperglycaemia and encouraged further investigation related to the molecular pathways of melatonin on glucose metabolism.
References
2. Maritim AC, Sanders A, Watkins JB (2003) Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 17 (1): 24-38. DOI: 10.1002/jbt.10058.
3. Al-Lawati JA (2017) Diabetes mellitus: a local and global public health emergency. Oman. Med. J. 32 (3): 177-179. DOI: 10.5001/omj.2017.34.
4. Pandey KB, Rizvi SI (2011) Biomarkers of oxidative stress in red blood cells. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. 155 (2): 131-136. DOI: 10.5507/bp.2011.027.
5. Asmat U, Abad K, Ismail K (2016) Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 24 (5): 547-553. DOI: 10.1016/j.jsps.2015.03.013.
6. Carelli-Alinovi C, Misiti F (2017) Erythrocytes as potential link between diabetes and Alzheimer’s disease. Front. Aging Neurosci. 9: 276. DOI: 10.3389/fnagi.2017.00276.
7. Angelousi A, Larger E (2015) Anaemia, a common but often unrecognized risk in diabetic patients: a review. Diabetes Metab. 41 (1): 18-27. DOI: 10.1016/j.diabet.2014.06.001.
8. Wang J, Wang H (2017) Oxidative stress in pancreatic beta cell regeneration. Oxid. Med. Cell. Longev. 2017:1930261. doi: 10.1155/2017/1930261.
9. Gerber PA, Rutter GA (2017) The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Sign. 26: 501–518. DOI: 10.1089/ars.2016.6755.
10. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51 (1): 1-6. DOI: 10.1111/j.1600-079X.2011.
11. Banerjee A, Chattopadhyay A, Pal PK, Bandyopadhyay D (2020) Melatonin is a potential therapeutic molecule for oxidative stress induced red blood cell (RBC) injury: A review. Melatonin Research 3 (1): 1-31. DOI: 10.32794/mr11250045.
12. Park JH, Shim HM, Na AY, Bae KC, Bae JH, Im SS, Cho HC, Song DK (2014) Melatonin prevents pancreatic β-cell loss due to glucotoxicity: the relationship between oxidative stress and endoplasmic reticulum stress. J. Pineal Res. 56 (2): 143-153. DOI: 10.1111/jpi.12106.
13. Jung KH, Hong SW, Zheng HM, Lee HS, Lee H, Lee DH, Lee SY, Hong SS (2010) Melatonin ameliorates cerulean-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J. Pineal Res. 48 (3): 239-250. DOI: 10.1111/j.1600-079X.2010.00748.x.
14. Champney TH, Brainard GC, Richardson BA, Reiter RJ (1983) Experimentally-induced diabetes reduces nocturnal pineal melatonin content in the Syrian hamster. Comp. Biochem. Phys. A. 76 (1): 199-201. DOI: 10.1016/0300-9629(83)90314-6.
15. Peschke E, Bähr I, Mühlbauer E (2013) Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon. Int. J. Mol Sci. 14 (4): 6981-7015. DOI: 10.3390/ijms14046981.
16. Rodríguez V, Mellado C, Alvarez E, De Diego JG, Blázquez E (1989) Effect of pinealectomy on liver insulin and glucagon receptor concentrations in the rat. J. Pineal Res. 6 (1): 77-88. DOI: 10.1111/j.1600-079x.1989.tb00405.x.
17. Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Schneyer U, Spessert R, Mühlbauer E (2006) Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J. Pineal Res. 40 (2): 135-143. DOI: 10.1111/j.1600-079X.2005.00287.x.
18. Rasmussen DD, Mitton DR, Larsen SA, Yellon SM (2001) Aging-dependent changes in the effect of daily melatonin supplementation on rat metabolic and behavioral responses. J. Pineal Res. 31 (1): 89-94. DOI: 10.1034/j.1600-079x.2001.310113.x.
19. Mulder H, Nagorny CL, Lyssenko V, Groop L (2009) Melatonin receptors in pancreatic islets: good morning to a novel type 2 diabetes gene. Diabetologia 52 (7): 1240-1249. DOI: 10.1007/s00125-009-1359-y.
20. Peschke E, Fauteck JD, Mußhoff U, Schmidt F, Beckmann A, Peschke D (2000) Evidence for a melatonin receptor within pancreatic islets of neonate rats: functional, autoradiographic, and molecular investigations. J. Pineal Res. 28 (3): 156-164. DOI: 10.1034/j.1600-079x.2001.280305.x.
21. Zephy D, Ahmad J. (2015) Type 2 diabetes mellitus: role of melatonin and oxidative stress. Diabetes Metab. Syndr. 9 (2): 127-131. DOI: 10.1016/j.dsx.2014.09.018.
22. Hevia D, González-Menéndez P, Quiros-González I, Miar A, Rodríguez-García A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: a new target for melatonin inhibition of cancer. J. Pineal Res. 58 (2): 234-250. DOI: 10.1111/jpi.12210.
23. Jones RL, Peterson CM (1981) Hematologic alterations in diabetes mellitus. Am. J. Med. 70 (2): 339-352. DOI: 10.1016/0002-9343(81)90771-3.
24. Huisjes R, Bogdanova A, van Solinge WW, Schiffelers RM, Kaestner L, Van Wijk R (2018) Squeezing for life–properties of red blood cell deformability. Front. Physiol. 9: 656. DOI: 10.3389/fphys.2018.00656.
25. Lux SE (1983) Disorders of the red cell membrane skeleton: hereditary spherocytosis and hereditary elliptocytosis. In: Metabolic basis of inherited disease, ed. Stanbury JB, 1573-1605, McGraw-Hill, New York.
26. Smith JE (1987) Erythrocyte membrane: structure, function, and pathophysiology. Vet. Pathol. 24 (6): 471-476. DOI: 10.1177/030098588702400601.
27. Wali RK, Jaffe S, Kumar D, Kalra VK (1988) Alterations in organization of phospholipids in erythrocytes as factor in adherence to endothelial cells in diabetes mellitus. Diabetes 37 (1): 104-111. DOI: 10.2337/diab.37.1.104.
28. Prisco D, Paniccia R, Coppo M, Vanni D, Rogasi PG, Tramontana M, Abbate R, Gensini GF (1989) Red blood cell lipid alterations in type II diabetes mellitus. Thromb. Res. 54 (6): 751-758. DOI: 10.1016/0049-3848(89)90139-4.
29. Buys AV, Van Rooy MJ, Soma P, Van Papendorp D, Lipinski B, Pretorius E (2013) Changes in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc. Diabetol. 12 (1). DOI: 10.1186/1475-2840-12-25.
30. Antonio PD, Lasalvia M, Perna G, Capozzi V (2012) Scale-independent roughness value of cell membranes studied by means of AFM technique. Biochim. Biophys. Acta Biomembranes. 1818 (12): 3141-3148. DOI: 10.1016/j.bbamem.2012.08.001.
31. Mazzanti L, Faloia E, Rabini RA, Staffolani R, Kantar A, Fiorini R, Swoboda B, de Pirro R, Bertoli E (1992) Diabetes mellitus induces red blood cell plasma membrane alterations possibly affecting the aging process. Clin. Biochem. 25 (1): 41-46. DOI: 10.1016/0009-9120(92)80044-h.
32. Testa I, Rabini RA, Fumelli P, Bertoli E, Mazzanti L (1988) Abnormal membrane fluidity and acetylcholinesterase activity in erythrocytes from insulin-dependent diabetic patients. J. Clin. Endocrinol. Metab. 67 (6): 1129-1133. DOI: 10.1210/jcem-67-6-1129.
33. Mawatari S, Saito K, Murakami K, Fujino T (2004) Absence of correlation between glycated hemoglobin and lipid composition of erythrocyte membrane in type 2 diabetic patients. Metab. 53 (1): 123-127. DOI: 10.1016/j.metabol.2003.07.016.
34. Maksina AG, Mikaelian NP, Kniazev I, Daĭniak BA (1992) Structural changes in erythrocyte membranes in diabetes mellitus using spin labelled fatty acids. Biofizika 37 (2): 306-309.
35. Forsyth AM, Braunmüller S, Wan J, Franke T, Stone HA (2012) The effects of membrane cholesterol and simvastatin on red blood cell deformability and ATP release. Microvasc. Res. 83 (3): 347-351. DOI: 10.1016/j.mvr.2012.02.004.
36. Baldini P, Incerpi S, Lambert-Gardini S, Spinedi A, Luly P (1989) Membrane lipid alterations and Na+-pumping activity in erythrocytes from IDDM and NIDDM subjects. Diabetes 38 (7): 825-831. DOI: 10.2337/diab.38.7.825.
37. Labrouche S, Freyburger G, Gin H, Boisseau MR, Cassagne C (1996) Changes in phospholipid composition of blood cell membranes (erythrocyte, platelet, and polymorphonuclear) in different types of diabetes—clinical and biological correlations. Metabolism 45 (1): 57-62. DOI: 10.1016/s0026-0495(96)90200-0.
38. Lipinski B, Pretorius E (2012) Novel pathway of iron-induced blood coagulation: implications for diabetes mellitus and its complications. Pol. Arch. Med. Wewn. 122: 115-122.
39. Pretorius E, Bester J, Vermeulen N, Alummoottil S, Soma P, Buys AV, Kell DB (2015) Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: implications for diagnostics. Cardiovasc. Diab. 14: 30. DOI: 10.1186/s12933-015-0192-5.
40. Rochette L, Zeller M, Cottin Y, Vergely C (2014) Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta General Subjects. 1840 (9): 2709-2729. DOI: 10.1016/j.bbagen.2014.05.017.
41. Kumar R (2012) Biochemical changes in erythrocyte membrane in type 2 diabetes mellitus. Indian J. Med. Sci. 66: 131-135. DOI: 10.4103/0019-5359.114199.
42. Eda S, Sherman I (2002) Cytoadherence of malaria-infected red blood cells involves exposure of phosphatidylserine. Cell. Physiol. Biochem. 12 (5-6): 373-384. DOI: 10.1159/000067908.
43. Kesavulu MM, Giri R, Rao BK, Apparao CH (2000) Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 26: 387-392.
44. Giugliano D, Ceriello A, Paolisso G (1995) Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress?. Metabolism 44 (3): 363-368. DOI: 10.1016/0026-0495(95)90167-1.
45. Jain SK, McVie R, Duett J, Herbst JJ (1989) Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 38 (12): 1539-1543. DOI: 10.2337/diab.38.12.1539.
46. Carrell RW, Winterbourn CC, Rachmilewitz EA (1975) Annotation: Activated oxygen and haemolysis. Br. J. Haematol. 30 (3): 259-264. DOI: 10.1111/j.1365-2141.1975.tb00540.x.
47. Ramasarma T. (1983) Generation of hydrogen peroxide in biomembranes. Biochim. Biophys. Acta. 694: 69–93. DOI: 10.1016/0304-4157(82)90014-4.
48. Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C (2010) Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J. Med. Assoc. Thai. 93 (6): 682-693.
49. Pazdro R, Burgess JR (2010) The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 131 (4): 276-286. DOI: 10.1016/j.mad.2010.03.005.
50. Taheri E, Djalali M, Saedisomeolia A, Moghadam AM, Djazayeri A, Qorbani M (2012) The relationship between the activates of antioxidant enzymes in red blood cells and body mass index in Iranian type 2 diabetes and healthy subjects. J. Diabetes Metab. Disord. 11 (1): 3. DOI: 10.1186/2251-6581-11-3.
51. Tesfamariam B (1994) Free radicals in diabetic endothelial cell dysfunction. Free Radic. Biol. Med. 16 (3): 383-391. DOI: 10.1016/0891-5849(94)90040-x.
52. Hamden K, Carreau S, Jamoussi K, Miladi S, Lajmi S, Aloulou D, Ayadi F, Elfeki A (2009) 1α, 25 dihydroxyvitamin D3: therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J. Nutr Sci. Vitaminol. 55 (3): 215-222. DOI: 10.3177/jnsv.55.215.
53. Wautier JL, Wautier MP, Schmidt AM, Anderson GM, Hori O, Zoukourian C, Capron L, Chappey O, Yan SD, Brett J (1994) Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc. Natl. Acad. Sci. 91 (16): 7742-7746. DOI: 10.1073/pnas.91.16.7742.
54. Shin S, Ku Y, Babu N, Singh M (2007) Erythrocyte deformability and its variation in diabetes mellitus. Indian J. Exp. Biol. 45: 121-128.
55. Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M (2005) Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 54 (11): 3274-3281. DOI: 10.2337/diabetes.54.11.3274.
56. Matkovics B, Varga SI, Szabo L, Witas H (1982) The effect of diabetes on the activities of the peroxide metabolism enzymes. Horm. Metab. Res. 14 (02): 77-79. DOI: 10.1055/s-2007-1018928.
57. Uzel N, Sivas A, Uysal M, Öz H (1987) Erythrocyte lipid peroxidation and glutathione peroxidase activities in patients with diabetes mellitus. Horm. Metab. Res. 19 (02): 89-90. DOI: 10.1055/s-2007-1011748.
58. Pretorius E (2013) The adaptability of red blood cells. Cardiovasc. Diab. 12: 63. DOI: 10.1186/1475-2840-12-63.
59. Wautier JL, Paton RC, Wautier MP, Pintigny D, Abadie E, Passa P, Caen JP (1981) Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications. N. Eng. J. Med. 305 (5): 237-242. DOI: 10.1056/NEJM198107303050501.
60. Schmid-Schönbein H, Volger E (1976) Red-cell aggregation and red-cell deformability in diabetes. Diabetes 25 (2 SUPPL): 897-902.
61. Grossin N, Wautier MP, Wautier JL (2009) Red blood cell adhesion in diabetes mellitus is mediated by advanced glycation end product receptor and is modulated by nitric oxide. Biorheology 46 (1): 63-72. DOI: 10.3233/BIR-2009-0519.
62. Wautier JL, LeBlanc H, Wautier MP, Abadie E, Passa P, Caen JP (1986) Erythrocyte adhesion to cultured endothelium and glycaemic control in Type I (insulin-dependent) diabetic patients. Diabetologia 29 (3): 151-155. DOI: 10.1007/bf02427085.
63. Berndt-Zipfel C, Michelson G, Dworak M, Mitry M, Löffler A, Pfützner A, Forst T (2013) Vildagliptin in addition to metformin improves retinal blood flow and erythrocyte deformability in patients with type 2 diabetes mellitus–results from an exploratory study. Cardiovasc. Diab. 12 (1): 59. DOI: 10.1186/1475-2840-12-59.
64. Jin H, Xing X, Zhao H, Chen Y, Huang X, Ma S, Ye H, Cai J (2010) Detection of erythrocytes influenced by aging and type 2 diabetes using atomic force microscope. Biochem. Biophys. Res. Commun. 391 (4): 1698-1702. DOI: 10.1016/j.bbrc.2009.12.133.
65. Basta G (2008) Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis 196 (1): 9-21. DOI: 10.1016/j.atherosclerosis.2007.07.025.
66. Engström G, Smith JG, Persson M, Nilsson PM, Melander O, Hedblad B (2014) Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J. Intern. Med. 276 (2): 174-183. DOI: 10.1111/joim.12188.
67. Hatanaka H, Hanyu H, Fukasawa R, Sato T, Shimizu S, Sakurai H (2016) Peripheral oxidative stress markers in diabetes-related dementia. Geriatr. Gerontol. Int. 16 (12): 1312-1318. DOI: 10.1111/ggi.12645.
68. Elkrief L, Rautou PE, Sarin S, Valla D, Paradis V, Moreau R (2016) Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 36 (7): 936-948. DOI: 10.1111/liv.13115.
69. Bonadonna RC, Bonora E, Del Prato S, Saccomani MP, Cobelli C, Natali A, Frascerra S, Pecori N, Ferrannini E, Bier D, DeFronzo RA (1996) Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes 45 (7): 915-925. DOI: 10.2337/diab.45.7.915.
70. Morabito R, Remigante A, Spinelli S, Vitale G, Trichilo V, Loddo S, Marino A (2020) High Glucose Concentrations Affect Band 3 Protein in Human Erythrocytes. Antioxidants 9 (5): 365. DOI: 10.3390/antiox9050365.
71. Astor BC, Muntner P, Levin A, Eustace JA, Coresh J (2002) Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988-1994). Arch. Intern. Med. 162 (12): 1401-1408. DOI: 10.1001/archinte.162.12.1401.
72. Singh DK, Winocour P, Farrington K (2009) Erythropoietic stress and anemia in diabetes mellitus. Nat. Rev. Endocrinol. 5 (4): 204-210. DOI: 10.1038/nrendo.2009.17.
73. Fava S, Azzopardi J, Ellard S, Hattersley AT (2001) ACE gene polymorphism as a prognostic indicator in patients with type 2 diabetes and established renal disease. Diabetes Care 24 (12): 2115-2120. DOI: 10.2337/diacare.24.12.2115.
74. Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J (2000) Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 26 (3): 163-177.
75. Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414 (6865): 813-820. DOI: 10.1038/414813a.
76. Rasouli N, Kern PA (2008) Adipocytokines and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 93 (11)_supplement_1: s64-73. DOI: 10.1210/jc.2008-1613.
77. Szoke E, Gerich JE (2005) Role of impaired insulin secretion and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Compr. Ther. 31 (2): 106-112. DOI: 10.1007/s12019-005-0005-y.
78. Benova TE, Viczenczova C, Bacova BS, Knezl V, Dosenko V, Rauchova H, Zeman M, Reiter RJ, Tribulova N (2019) Obesity-associated alterations in cardiac connexin-43 and PKC signaling are attenuated by melatonin and omega-3 fatty acids in female rats. Mol. Cell Biochem. 454 (1-2): 191-202. DOI: 10.1007/s11010-018-3463-0.
79. Ríos-Lugo MJ, Cano P, Jiménez-Ortega V, Fernández-Mateos MP, Scacchi PA, Cardinali DP, Esquifino AI (2010) Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat–fed rats. J. Pineal Res. 49 (4): 342-348. DOI: 10.1111/j.1600-079X.2010.00798.x.
80. Agil A, Rosado I, Ruiz R, Figueroa A, Zen N, Fernández-Vázquez G (2012) Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J. Pineal Res. 52 (2): 203-210. DOI: 10.1111/j.1600-079X.2011.00928.x.
81. Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, Marchal JA, Adem A, Fernández-Vázquez G (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 54 (4): 381-388. DOI: 10.1111/jpi.12012.
82. Gustafson B (2010) Adipose tissue, inflammation and atherosclerosis. J. Atheroscler. Thromb. 17 (4): 332-341. DOI: 10.5551/jat.3939.
83. Negi G, Kumar A, Sharma SS (2011) Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-κB and Nrf2 cascades. J. Pineal Res. 50 (2): 124-131. DOI: 10.1111/j.1600-079X.2010.00821.x.
84. Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ. Res. 107 (9): 1058-1070. DOI: 10.1161/CIRCRESAHA.110.223545.
85. Wei YH, Lee HC (2002) Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp. Biol. Med. 227 (9): 671-682. DOI: 10.1177/153537020222700901.
86. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B (1996) Evaluation of the antioxidant activity of melatonin in vitro. Free Radic. Biol. Med. 21 (3): 307-315. DOI: 10.1016/0891-5849(96)00046-9.
87. Salmanoglu DS, Gurpinar T, Vural K, Ekerbicer N, Darıverenli E, Var A (2016) Melatonin and L-carnitin improves endothelial disfunction and oxidative stress in Type 2 diabetic rats. Redox Biol. 8: 199-204. DOI: 10.1016/j.redox.2015.11.007.
88. Rybka J, Kędziora-Kornatowska K, Kupczyk D, Muszalik M, Kornatowski M, Kędziora J (2016) Antioxidant effect of immediate-versus sustained-release melatonin in type 2 diabetes mellitus and healthy controls. Drug Deliv. 23 (3): 804-807. DOI: 10.3109/10717544.2014.917343.
89. Marjani A (2010) Lipid peroxidation alterations in type 2 diabetic patients. Pak. J. Biol. Sci. 13 (15): 723-730. DOI: 10.3923/pjbs.2010.723.730.
90. Vural H, Sabuncu T, Arslan SO, Aksoy N (2001) Melatonin inhibits lipid peroxidation and stimulates the antioxidant status of diabetic rats. J. Pineal Res. 31 (3): 193-198. DOI: 10.1034/j.1600-079x.2001.310301.x.
91. Bundhun PK, Bhurtu A, Yuan J (2017) Impact of type 2 diabetes mellitus on the long-term mortality in patients who were treated by coronary artery bypass surgery: a systematic review and meta-analysis. Medicine 96 (22). DOI: 10.1097/MD.0000000000007022.
92. Bonamichi BD, Parente EB, Campos AC, Cury AN, Salles JE (2017) Hyperglycemia effect on coronary disease in patients with metabolic syndrome evaluated by intracoronary ultrasonography. PloS one 12 (2). DOI: 10.1371/journal.pone.0171733.
93. Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z (2019) Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 38 (1): 191-196. DOI: 10.1016/j.clnu.2017.12.004.
94. Alamdari NM, Mahdavi R, Roshanravan N, Yaghin NL, Ostadrahimi AR, Faramarzi E (2015) A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm. Metab. Res. 47 (07): 504-508. DOI: 10.1055/s-0034-1384587.
95. Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M (2011) Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 50 (3): 261-266. DOI: 10.1111/j.1600-079X.2010.00835.x.
96. Urata Y, Honma S, Goto S, Todoroki S, Iida T, Cho S, Honma K, Kondo T (1999) Melatonin induces γ-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 27 (7-8): 838-847. DOI: 10.1016/s0891-5849(99)00131-8.
97. Fex M, Nicholas LM, Vishnu N, Medina A, Sharoyko VV, Nicholls DG, Spégel P, Mulder H (2018) The pathogenetic role of β-cell mitochondria in type 2 diabetes. J. Endocrinol. 236 (3): R145-159. DOI: 10.1530/JOE-17-0367.
98. R Ramis M, Esteban S, Miralles A, Tan DX, J Reiter R (2015) Protective effects of melatonin and mitochondria-targeted antioxidants against oxidative stress: A review. Curr. Med. Chem. 22 (22): 2690-2711. DOI: 10.2174/0929867322666150619104143.
99. ABDEL-WAHAB MH, ABD-ALLAH AR (2000) Possible protective effect of melatonin and/or desferrioxamine against streptozotocin-induced hyperglycaemia in mice. Pharmacol. Res. 41 (5): 533-537. DOI: 10.1006/phrs.1999.0614.
100. Pierrefiche G, Topall G, Courboin G, Henriet I, Laborit H (1993) Antioxidant activity of melatonin in mice. Res. Commun. Chem. Pathol. Pharmacol. 80 (2): 211-223.
101. Anwar MM, Meki AR (2003) Oxidative stress in streptozotocin-induced diabetic rats: effects of garlic oil and melatonin. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 135 (4): 539-547. DOI: 10.1016/s1095-6433(03)00114-4.
102. Damasceno DC, Volpato GT, Calderon ID, Rudge MV (2002) Oxidative stress and diabetes in pregnant rats. Anim. Reprod. Sci. 72 (3-4): 235-244. DOI: 10.1016/s0378-4320(02)00094-5.
103. Gul M, Laaksonen DE, Atalay M, Vider L, Hänninen O (2002) Effects of endurance training on tissue glutathione homeostasis and lipid peroxidation in streptozotocin-induced diabetic rats. Scand. J. Med. Sci. Spor. 12 (3): 163-170. DOI: 10.1034/j.1600-0838.2002.120307.x.
104. Kędziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kędziora J, Bartosz G (2002) The effect of verapamil on the antioxidant defence system in diabetic kidney. Clin. Chim. Acta. 322 (1-2): 105-112. DOI: 10.1016/s0009-8981(02)00167-5.
105. Baydas G, Canatan H, Turkoglu A (2002) Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J. Pineal Res. 32 (4): 225-230. DOI: 10.1034/j.1600-079x.2002.01856.x.
106. Montilla PL, Vargas JF, Túnez IF, Carmen M, de Agueda M, Valdelvira ME, Cabrera ES (1998) Oxidative stress in diabetic rats induced by streptozotocin: protective effects of melatonin. J. Pineal Res. 25 (2): 94-100. DOI: 10.1111/j.1600-079x.1998.tb00545.x.
107. Şekeroğlu MR, Huyut Z, Çokluk E, Özbek H, Alp HH (2017) The susceptibility to autoxidation of erythrocytes in diabetic mice: Effects of melatonin and pentoxifylline. J. Biochem. Mol. Toxicol. 31 (12): e21976. DOI: 10.1002/jbt.21976.
108. Sailaja Devi MM, Suresh Y, Das UN (2000) Preservation of the antioxidant status in chemically-induced diabetes mellitus by melatonin. J. Pineal Res. 29 (2): 108-115. DOI: 10.1034/j.1600-079x.2000.290207.x.
109. Kvetnoy IM (1999) Extrapineal melatonin: location and role within diffuse neuroendocrine system. Histochem. J. 31 (1): 1-2. DOI: 10.1023/a:1003431122334.
110. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell. Mol. Life Sci. 71 (16): 2997-3025. DOI: 10.1007/s00018-014-1579-2.
111. Knutson KL, Ryden AM, Mander BA, Van Cauter E (2006) Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch. Intern. Med. 166 (16): 1768-1774. DOI: 10.1001/archinte.166.16.1768.
112. Milcu SM, Nanu-Ionescu L, Milcu I (1971) The effect of pinealectomy on plasma insulin in rats. In: Ciba Foundation Symposium-Ageing in Transient Tissues (Colloquia on Ageing) (pp. 345-360). Chichester, UK: John Wiley & Sons, Ltd.
113. Spiegel K, Knutson K, Leproult R, Tasali E, Cauter EV (2005) Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J. Appl. Physiol. 99 (5): 2008-2019. DOI: 10.1152/japplphysiol.00660.2005.
114. Hikichi T, Tateda N, Miura T (2011) Alteration of melatonin secretion in patients with type 2 diabetes and proliferative diabetic retinopathy. Clin. Ophthalmol. 5: 655- 660. DOI: 10.2147/OPTH.S19559.
115. Namboodiri MA, Favilla JT, Klein DC (1981) Pineal N-acetyltransferase is inactivated by disulfide-containing peptides: insulin is the most potent. Science 213 (4507): 571-573. DOI: 10.1126/science.7017937.
116. Csaba G, Barath P (1971) Are Langerhans's islets influenced by the pineal body?. Experientia 27 (8): DOI: 10.1007/bf02135774.
117. Nishida S, Sato R, Murai I, Nakagawa S (2003) Effect of pinealectomy on plasma levels of insulin and leptin and on hepatic lipids in type 2 diabetic rats. J. Pineal Res. 35 (4): 251-256. DOI: 10.1034/j.1600-079x.2003.00083.x.
118. Tutuncu NB, Batur MK, Yildirir A, Tutuncu T, Deger A, Koray Z, Erbas B, Kabakci G, Aksoyek S, Erbas T (2005) Melatonin levels decrease in type 2 diabetic patients with cardiac autonomic neuropathy. J. Pineal Res. 39 (1): 43-49. DOI: 10.1111/j.1600-079X.2005.00213.x.
119. Boden GU, Ruiz JO, Urbain JL, Chen XI (1996) Evidence for a circadian rhythm of insulin secretion. Am. J. Physiol. Endocrinol. Metab. 271 (2): E246-252. DOI: 10.1152/ajpendo.1996.271.2.E246.
120. Costes S, Boss M, Thomas AP, Matveyenko AV (2015) Activation of melatonin signaling promotes β-cell survival and function. Mol. Endocrinol. 29 (5): 682-692. DOI: 10.1210/me.2014-1293.
121. Karamitri A, Renault N, Clement N, Guillaume JL, Jockers R (2013) Minireview: Toward the establishment of a link between melatonin and glucose homeostasis: association of melatonin MT2 receptor variants with type 2 diabetes. Mol. Endocrinol. 27 (8): 1217-1233. DOI: 10.1210/me.2013-1101.
122. Kemp DM, Ubeda M, Habener JF (2002) Identification and functional characterization of melatonin Mel 1a receptors in pancreatic β cells: potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol. Cell Endocrinol. 191 (2): 157-166. DOI: 10.1016/s0303-7207(02)00064-3.
123. Peschke E, Mühlbauer E, Mußhoff U, Csernus VJ, Chankiewitz E, Peschke D (2002) Receptor (MT1) mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J. Pineal Res. 33 (2): 63-71. DOI: 10.1034/j.1600-079x.2002.02919.x.
124. Mühlbauer E, Peschke E (2007) Evidence for the expression of both the MT1-and in addition, the MT2-melatonin receptor, in the rat pancreas, islet and β-cell. J. Pineal Res. 42 (1): 105-106. DOI: 10.1111/j.1600-079X.2006.00399.x.
125. Ramracheya RD, Muller DS, Squires PE, Brereton H, Sugden D, Huang GC, Amiel SA, Jones PM, Persaud SJ (2008) Function and expression of melatonin receptors on human pancreatic islets. J. Pineal Res. 44 (3): 273-279. DOI: 10.1111/j.1600-079X.2007.00523.x.
126. Mulder H, Nagorny CL, Lyssenko V, Groop L (2009) Melatonin receptors in pancreatic islets: good morning to a novel type 2 diabetes gene. Diabetologia 52 (7): 1240-1249. DOI: 10.1007/s00125-009-1359-y.
127. Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF (1995) Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc. Natl. Acad. Sci. 92 (19): 8734-8738. DOI: 10.1073/pnas.92.19.8734.
128. Mühlbauer E, Albrecht E, Bazwinsky-Wutschke I, Peschke E (2012) Melatonin influences insulin secretion primarily via MT1 receptors in rat insulinoma cells (INS‐1) and mouse pancreatic islets. J. Pineal Res. 52 (4): 446-459. DOI: 10.1111/j.1600-079X.2012.00959.x.
129. Contreras-Alcantara S, Baba K, Tosini G (2010) Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity 18 (9): 1861-1863. DOI: 10.1038/oby.2010.24.
130. Tuomi T, Nagorny CL, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Östman B, Söderström J, Pesonen AK, Martikainen S, Räikkönen K, Forsén T, Hakaste L, Almgren P, Storm P, Asplund O, Shcherbina L, Fex M, Fadista J, Tengholm A, Wierup N, Groop L, Mulder H (2016) Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 23 (6): 1067-1077. DOI: 10.1016/j.cmet.2016.04.009.
131. Karamitri A, Plouffe B, Bonnefond A, Chen M, Gallion J, Guillaume JL, Hegron A, Boissel M, Canouil M, Langenberg C, Wareham NJ (2018) Type 2 diabetes–associated variants of the MT2 melatonin receptor affect distinct modes of signaling. Sci. Signal. 11 (545): eaan6622. DOI: 10.1126/scisignal.aan6622.
132. Bonnefond A, Clément N, Fawcett K, Yengo L, Vaillant E, Guillaume JL, Dechaume A, Payne F, Roussel R, Czernichow S, Hercberg S, Hadjadj S, Balkau B, Marre M, Lantieri O, Langenberg C, Bouatia-Naji N, Charpentier G, Vaxillaire M, Rocheleau G, Wareham NJ, Sladek R, McCarthy MI, Dina C, Barroso I, Jockers R, Froguel P (2012) Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat. Genet. 44 (3): 297. DOI: 10.1038/ng.1053.
133. Semiz S, Dujic T, Velija-Asimi Z, Prnjavorac B, Bego T, Ostanek B, Marc J, Causevic A (2014) Effects of melatonin receptor 1B gene variation on glucose control in population from Bosnia and Herzegovina. Exp. Clin. Endocrinol. Diabetes 122 (06): 350-355. DOI: 10.1055/s-0034-1371871.
134. Dezaki K, Kakei M, Yada T (2007) Ghrelin uses Gαi2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes 56 (9): 2319-2327. DOI: 10.2337/db07-0345.
135. Karamitri A, Jockers R (2019) Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 15 (2): 105-125. DOI: 10.1038/s41574-018-0130-1.
136. Peschke E, Mühlbauer E (2010) New evidence for a role of melatonin in glucose regulation. Best Pract. Res. Clin. Endocrinol. Metab. 24 (5): 829-841. DOI: 10.1016/j.beem.2010.09.001.
137. Petit L, Lacroix I, de Coppet P, Strosberg AD, Jockers R (1999) Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′-5′-monophosphate pathway. Biochem. Pharmacol. 58 (4): 633-639. DOI: 10.1016/s0006-2952(99)00134-3.
138. Stumpf I, Mühlbauer E, Peschke E (2008) Involvement of the cGMP pathway in mediating the insulin-inhibitory effect of melatonin in pancreatic β-cells. J. Pineal Res. 45 (3): 318-327. DOI: 10.1111/j.1600-079X.2008.00593.x.
139. Brydon L, Roka F, Petit L, de Coppet P, Tissot M, Barrett P, Morgan PJ, Nanoff C, Strosberg AD, Jockers R (1999) Dual signaling of human Mel1a melatonin receptors via Gi2, Gi3, and Gq/11 proteins. Mol. Endocrinol. 13 (12): 2025-2038. DOI: 10.1210/mend.13.12.0390.
140. Peschke E, Bach AG, Mühlbauer E (2006) Parallel signaling pathways of melatonin in the pancreatic β-cell. J. Pineal Res. 40 (2): 184-191. DOI: 10.1111/j.1600-079X.2005.00297.x.
141. Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41 (1): 82-88. DOI: 10.1038/ng.288.
142. Simsek N, Kaya M, Kara A, Can I, Karadeniz A, Kalkan Y (2012) Effects of melatonin on islet neogenesis and beta cell apoptosis in streptozotocin-induced diabetic rats: an immunohistochemical study. Domest. Anim. Endocrinol. 43 (1): 47-57. DOI: 10.1016/j.domaniend.2012.02.002.
143. Reiter RJ, Paredes SD, Manchester LC, Tan DX (2009) Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 44 (4): 175-200. DOI: 10.1080/10409230903044914.
144. She M, Laudon M, Yin W (2014) Melatonin receptors in diabetes: a potential new therapeutical target? Eur. J. Pharmacol. 744: 220-223. DOI: 10.1016/j.ejphar.2014.08.012.
145. Peschke E, Bähr I, Mühlbauer E (2015) Experimental and clinical aspects of melatonin and clock genes in diabetes. J. Pinael Res. 59 (1): 1-23. DOI: 10.1111/jpi.12240.
146. Pernow J, Mahdi A, Yang J, Zhou Z (2019) Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc. Res. 115 (11): 1596-1605. DOI: 10.1093/cvr/cvz156.
147. Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Gladwin MT (2005) Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 294 (1): 81-90. DOI: 10.1001/jama.294.1.81.
148. Catan A, Turpin C, Diotel N, Patche J, Guerin-Dubourg A, Debussche X, Bourdon E, Ah-You N, Le Moullec N, Besnard M, Veerapen R (2019) Aging and glycation promote erythrocyte phagocytosis by human endothelial cells: Potential impact in atherothrombosis under diabetic conditions. Atherosclerosis 291: 87-98. DOI: 10.1016/j.atherosclerosis.2019.10.015.
149. Gambhir KK, Archer JA, Bradley CJ (1978) Characteristics of human erythrocyte insulin receptors. Diabetes 27 (7): 701-708. DOI: 10.2337/diab.27.7.701.
150. Eaton RP, Galagan R, Kaufman E, Allen RC, Russell L, Miller F (1981) Receptor depletion in diabetes mellitus: correction with therapy. Diabetes Care 4 (2): 299-304. DOI: 10.2337/diacare.4.2.299.
151. Zancan P, Sola-Penna M (2005) Regulation of human erythrocyte metabolism by insulin: cellular distribution of 6-phosphofructo-1-kinase and its implication for red blood cell function. Mol. Genet. Metab. 86 (3): 401-411. DOI: 10.1016/j.ymgme.2005.06.011.
152. Scionti L, Puxeddu A, Calabrese G, Gatteschi C, De Angelis M, Bolli G, Compagnucci P, Calafiore R, Brunetti P (1982) Erythrocyte concentration of glycolytic phosphorylated intermediates and adenosine nucleotides in subjects with diabetes mellitus. Horm. Metab. Res. 14 (05): 233-236. DOI: 10.1055/s-2007-1018980.
153. Suhail M, Rizvi S (1989) Effect of type I (insulin-dependent) diabetes mellitus on key glycolytic enzymes of red blood cells. Acta Diabetol. Lat. 26 (4): 315-320. DOI: 10.1007/bf02624643.
154. Chapman RG, Schaumburg L (1967) Glycolysis and glycolytic enzyme activity of aging red cells in man: changes in hexokinase, aldolase, glyceraldehyde-3-phosphate dehydrogenase, pyruvate kinase and glutamic-oxalacetic transaminase. Br. J. Haematol. 5: 665-678. DOI: 10.1111/j.1365-2141.1967.tb08832.x.
155. Pescarmona GP, Bosia A, Ghigo D (1982) Shortened red cell life span in diabetes: mechanism of hemolysis. In: Advances in red cell biology (pp. 391-397). Raven New York, NY.
156. Mayo JC, Aguado A, Cernuda-Cernuda R, Álvarez-Artime A, Cepas V, Quirós-González I, Hevia D, Sáinz RM (2018) Melatonin uptake by cells: an answer to its relationship with glucose? Molecules 23 (8): E1999. DOI: 10.3390/molecules23081999.
157. García JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter RJ, Ramírez JM, Bernal-Pérez M (2014) Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J. Pineal Res. 56 (3): 225-237. DOI: 10.1111/jpi.12128.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


