Melatonin as a prospective metabolic regulator in pathologically altered cardiac energy homeostasis
Melatonin and cardiac metabolism
Abstract
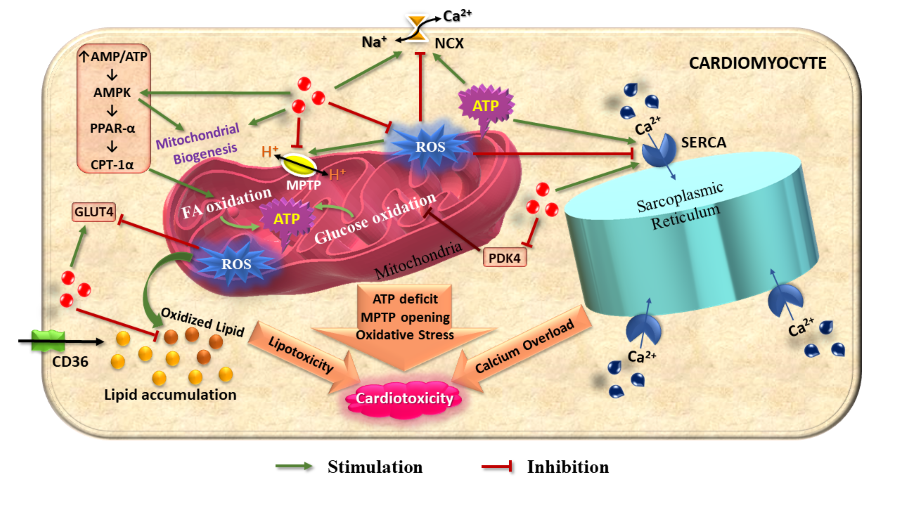
A constant energy supply is indispensable for the relentlessly working heart. The unique metabolic flexibility of the cardiac tissue enables it to maintain its energy requirement under variable physiological conditions. However, some physiopathological statuses including aging, ischemia-reperfusion injury, diabetic cardiomyopathy, pathological cardiac hypertrophy, and heart failure frequently cause cardiac dysfunction and detrimental metabolic alteration. If the ATP supply fails to match the requirement of a working heart, the heart loses its functional capacity, resulting in slower recovery. A decrease in energy generation is often the ramifications of myocardial mitochondrial dysfunction and oxidative stress. Melatonin, a broad-spectrum antioxidant molecule has an appreciable role in the maintenance of metabolic homeostasis― from a single cell to an entire organism. Melatonin has the capacity to reduce ROS generation, preserve mitochondrial stability, and restore a robust mitochondrial function for unabated ATP production in cardiac tissues. Additionally, melatonin can promote carbohydrate and fat metabolism to further improve the ATP production in heart. In cardiac cells, melatonin upregulates GLUT4 expression either by impeding oxidative stress or by enhancing AMPK activation which accelerates fatty acid oxidation by upregulating PPAR-α and CPT-1α. Melatonin plays a pivotal role in the maintenance of calcium homeostasis in cardiomyocytes by obviating oxidative stress-mediated disruption of SERCA and NCX proteins. A possible role of melatonin to convert the Warburg effect to oxidative metabolism in pathological cardiac events has been recently contemplated. The current review will discuss the possible role of melatonin protecting against cardiac metabolic imbalances under pathological states.
References
2. Gibb AA, Hill BG (2018) Metabolic coordination of physiological and pathological cardiac remodeling. Circ. Res. 123: 107-128. DOI:10.1161/CIRCRESAHA.118.312017.
3. Battaglia FC, Meschia G (1978) Principal substrates of fetal metabolism. Physiol. Rev. 58: 499–527. DOI: 10.1152/physrev.1978.58.2.499.
4. Makinde AO, Kantor PF, Lopaschuk GD (1998) Maturation of fatty acid and carbohydrate metabolism in the newborn heart. Mol. Cell. Biochem. 188: 49–56.
5. Kaijser L, Berglund B (1992) Myocardial lactate extraction and release at rest and during heavy exercise in healthy men. Acta Physiol. Scand. 144: 39–45. DOI: 10.1111/j.1748-1716.1992.tb09265.x.
6. Rider OJ, Lewis AJ, Neubauer S (2014) Structural and metabolic effects of obesity on the myocardium and the aorta. Obes. Facts. 7: 329-338. DOI:10.1159/000368429.
7. Bayeva M, Sawicki KT, Ardehali H (2013) Taking diabetes to heart―deregulation of myocardial lipid metabolism in diabetic cardiomyopathy. J. Am. Heart Assoc. 2: e000433. Published 2013 Nov 25. doi:10.1161/JAHA.113.000433.
8. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90: 207-258. DOI:10.1152/physrev.00015.2009.
9. Sun H, Gusdon AM, Qu S (2016) Effects of melatonin on cardiovascular diseases: progress in the past year. Curr. Opin. Lipidol. 27: 408-413. DOI: 10.1097/MOL.0000000000000314.
10. Pandi-Perumal SR, BaHammam AS, Ojike NI, Akinseye OA, Kendzerska T, Buttoo K, Dhandapany PS, Brown GM, Cardinali DP (2017) Melatonin and human cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 22: 122-132. DOI:10.1177/1074248416660622.
11. Sanchez-Hidalgo M, de la Lastra CA, Carrascosa-Salmoral MP, Naranjo MC, Gomez-Corvera A, Caballero B, Guerrero JM (2009) Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 44: 328-334. DOI: 10.1016/j.exger.2009.02.002.
12. Korkmaz A, Topal T, Tan DX, Reiter RJ (2009) Role of melatonin in metabolic regulation. Rev. Endocr. Metab. Disord. 10: 261-270. DOI:10.1007/s11154-009-9117-5.
13. Krause DN, Geary GG, Doolen S, Duckles SP (2002) Melatonin and cardiovascular function. in melatonin after four decades. (Springer, Boston, MA), pp 299-310. DOI: https://doi.org/10.1007/0-306-46814-X_32.
14. Girotti L, Lago M, Ianovsky O, Carbajales J, Elizari MV, Brusco LI, Cardinali DP (2000) Low urinary 6‐sulphatoxymelatonin levels in patients with coronary artery disease. J. Pineal Res. 29: 138-142. DOI: 10.1034/j.1600-079x.2000.290302.x.
15. Ritterhoff J, Tian R (2017) Metabolism in cardiomyopathy: every substrate matters. Cardiovasc. Res. 113: 411-421. DOI:10.1093/cvr/cvx017.
16. Scolletta S, Biagioli B (2010) Energetic myocardial metabolism and oxidative stress: let's make them our friends in the fight against heart failure. Biomed. Pharmacother. 64: 203-207. DOI:10.1016/j.biopha.2009.10.002.
17. Pascual F, Coleman RA (2016) Fuel availability and fate in cardiac metabolism: A tale of two substrates. Biochim. Biophys. Acta. 1861: 1425-1433. DOI:10.1016/j.bbalip.2016.03.014.
18. Patterson AJ, Zhang L (2010) Hypoxia and fetal heart development. Curr. Mol. Med. 10: 653-666. DOI:10.2174/156652410792630643.
19. Semenza GL (2014) Hypoxia-inducible factor 1 and cardiovascular disease. Annu. Rev. Physiol. 76: 39–56.
20. Pederson BA, Chen H, Schroeder JM, Shou W, DePaoli-Roach AA, Roach PJ (2004) Abnormal cardiacdevelopment in the absence of heart glycogen. Mol. Cell. Biol. 24: 7179–7187.
21. Laird-Meeter K, van de Ley G, Bom TH, Wladimiroff JW, Roelandt J (1979) Cardiocirculatory adjustments during pregnancy― an echocardiographic study. Clin. Cardiol. 2: 328–332.
22. Hunter S, Robson SC (1992) Adaptation of the maternal heart in pregnancy. Br. Heart J. 68: 540–543.
23. Umar S, Nadadur R, Iorga A, Amjedi M, Matori H, Eghbali M(2012) Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J. Appl. Physiol. 113: 1253–1259. DOI: 10.1152/japplphysiol.00549.2012.
24. Sugden MC, Changani KK, Bentley J, Holness MJ (1992) Cardiac glucose metabolism during pregnancy. Biochem. Soc. Trans. 20: 195S.
25. Metzger BE, Hare JW, Freinkel N (1971) Carbohydrate metabolism in pregnancyIX. Plasma levels of gluconeogenic fuels during fasting in the rat. J. Clin. Endocrinol. Metab. 33: 869–872. DOI: 10.1210/jcem-33-5-869.
26. Pinto J, Barros AS, Domingues MR, Goodfellow BJ, Galhano E, Pita C, Almeida Mdo C, Carreira IM, Gil AM (2015) Following healthy pregnancy by NMR metabolomics of plasma and correlation to urine. J. Proteome Res. 14: 1263–1274. DOI: 10.1021/pr5011982.
27. Liu LX, Rowe GC, Yang S, Li J, Damilano F, Chan MC, Lu W, Jang C, Wada S, Morley M, Hesse M (2017) PDK4 inhibits cardiac pyruvate oxidation in late pregnancy. Circ. Res. 121: 1370–1378. DOI: 10.1161/CIRCRESAHA.117.311456.
28. Goodwin GW, Taylor CS, Taegtmeyer H (1998) Regulation of energy metabolism of the heart during acute increase in heart work. J. Biol. Chem. 273: 29530–29539.
29. Goodwin GW, Taegtmeyer H (2000) Improved energy homeostasis of the heart in the metabolic state of exercise. Am. J. Physiol. Heart Circ. Physiol. 279: H1490–H1501. DOI: 10.1152/ajpheart.2000.279.4.H1490.
30. Gousios A, Felts JM, Havel RJ (1965) Effect of catecholamines, glucose, insulin, and changes of flow on the metabolism of free fatty acids by the myocardium. Metabolism 14: 826–831.
31. Buse MG, Biggers JF, Drier C, Buse JF (1973) The effect of epinephrine, glucagon, and the nutritional state on the oxidation of branched chain amino acids and pyruvate by isolated hearts and diaphragms of the rat. J. Biol. Chem. 248: 697–706.
32. Crass MF III, Shipp JC, Pieper GM (1975) Effects of catecholamines on myocardial endogenous substrates and contractility. Am. J. Physiol. 228: 618–627. DOI: 10.1152/ajplegacy.1975.228.2.618.
33. Collins-Nakai RL, Noseworthy D, Lopaschuk GD (1994) Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. Am. J. Physiol. 267: H1862–H1871. DOI: 10.1152/ajpheart.1994.267.5.H1862.
34. Clark MG, Patten GS (1981) Adrenaline activation of phosphofructokinase in rat heart mediated by alpha-receptor mechanism independent of cyclic AMP. Nature 292: 461–463.
35. Lassers BW, Kaijser L, Wahlqvist M, Carlson LA (1971) Effect of prolonged exercise on myocardial metabolism in man. Br. Heart J. 33: 609.
36. Donath MY, Jenni R, Brunner HP, Anrig M, Kohli S, Glatz Y, Froesch ER (1996) Cardiovascular and metabolic effects of insulin-like growth factor I at rest and during exercise in humans. J. Clin. Endocrinol. Metab. 81: 4089–4094. DOI: 10.1210/jcem.81.11.8923865.
37. Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH (1997) Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J. Biol. Chem. 272: 17269–17275.
38. Pentassuglia L, Heim P, Lebboukh S, Morandi C, Xu L, Brink M (2016) Neuregulin-1β promotes glucose uptake via PI3K/Akt in neonatal rat cardiomyocytes. Am. J. Physiol. Endocrinol. Metab. 310: E782–E794. DOI: 10.1152/ajpendo.00259.2015.
39. Dolinsky VW, Dyck JR (2006) Role of AMP-activated protein kinase in healthy and diseased hearts. Am. J. Physiol. Heart Circ. Physiol. 291: H2557–H2569. DOI: 10.1152/ajpheart.00329.2006.
40. Yavari A, Bellahcene M, Bucchi A, Sirenko S, Pinter K, Herring N, Jung JJ, Tarasov KV, Sharpe EJ, Wolfien M, Czibik G, Steeples V, Ghaffari S, Nguyen C, Stockenhuber A, Clair JRS, Rimmbach C, Okamoto Y, Yang D, Wang M, Ziman BD, Moen JM, Riordon DR, Ramirez C, Paina M, Lee J, Zhang J, Ahmet I, Matt MG, Tarasova YS, Baban D, Sahgal N, Lockstone H, Puliyadi R, de Bono J, Siggs OM, Gomes J, Muskett H, Maguire ML, Beglov Y, Kelly M, Dos Santos PPN, Bright NJ, Woods A, Gehmlich K, Isackson H, Douglas G, Ferguson DJP, Schneider JE, Tinker A, Wolkenhauer O, Channon KM, Cornall RJ, Sternick EB, Paterson DJ, Redwood CS, Carling D, Proenza C, David R, Baruscotti M, DiFrancesco D, Lakatta EG, Watkins H, Ashrafian H (2017) Mammalian γ2 AMPK regulates intrinsic heart rate. Nat. Commun. 8: 1258. DOI: 10.1038/s41467-017-01342-5.
41. Sen S, Kundu BK, Wu HC, Hashmi SS, Guthrie P, Locke LW, Roy RJ, Matherne GP, Berr SS, Terwelp M, Scott B, Carranza S, Frazier OH, Glover DK, Dillmann WH, Gambello MJ, Entman ML, Taegtmeyer H (2013) Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J. Am. Heart Assoc. 2: e004796. DOI: 10.1161/JAHA.113.004796.
42. Lesnefsky EJ, Chen Q, Hoppel CL (2016) Mitochondrial metabolism in aging heart. Circ. Res. 118: 1593-1611. DOI:10.1161/CIRCRESAHA.116.307505.
43. Hyyti OM, Ledee D, Ning XH, Ge M, Portman MA (2010) Aging impairs myocardial fatty acid and ketone oxidation and modifies cardiac functional and metabolic responses to insulin in mice. Am. J. Physiol. Heart Circ. Physiol. 299: H868–875.
44. Moreau R, Heath SH, Doneanu CE, Harris RA, Hagen TM (2004) Age-related compensatory activation of pyruvate dehydrogenase complex in rat heart. Biochem. Biophys. Res. Commun. 325: 48–58.
45. Koonen DP, Febbraio M, Bonnet S, Nagendran J, Young ME, Michelakis ED, Dyck JR (2007) Cd36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 116: 2139–2147.
46. Allard MF. Energy substrate metabolism in cardiac hypertrophy (2004) Curr. Hypertens. Rep. 6: 430-435. DOI:10.1007/s11906-004-0036-2.
47. Swyngedhauw B (1999) Molecular mechanisms of myocardial remodeling. Physiol. Rev. 79: 215–262.
48. Gaasch WH, Zile MR, Hoshino PK, Weinberg EO, Rhodes DR, Apstein CS (1990) Tolerance of the hypertrophic heart to ischemia. Studies in compensated and failing dog hearts with pressure overload hypertrophy. Circulation 81: 1644-1653. DOI:10.1161/01.cir.81.5.1644.
49. Stanley WC, Lopaschuk GD, Hall JL, McCormack JG (1997) Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc. Res. 33: 243-257. DOI:10.1016/s0008-6363(96)00245-3.
50. Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF (2002) Energy metabolism in the hypertrophied heart. Heart Fail. Rev. 7: 161-173. DOI:10.1023/a:1015380609464.
51. Siddiqi N, Singh S, Beadle R, Dawson D, Frenneaux M (2013) Cardiac metabolism in hypertrophy and heart failure: implications for therapy. Heart Fail. Rev. 18: 595-606. DOI:10.1007/s10741-012-9359-2.
52. Lopaschuk GD (2016) Metabolic changes in the acutely ischemic heart. Heart Metab. 70: 32-35.
53. Rosano GM, Fini M, Caminiti G, Barbaro G (2008) Cardiac metabolism in myocardial ischemia. Curr. Pharm. Des. 14: 2551-2562. DOI:10.2174/138161208786071317.
54. Halestrap AP (2009) What is the mitochondrial permeability transition pore?. J. Mol. Cell Cardiol. 46: 821-831. DOI:10.1016/j.yjmcc.2009.02.021.
55. Neely JR, Rovetto MJ, Whitmer JT, Morgan HE (1973) Effects of ischemia on function and metabolism of the isolated working rat heart. Am. J. Physiol. 225: 651-658.
56. Hochachka PW, Mommsen TP (1983) Protons and anaerobiosis. Science 219: 1391-1397.
57. Hendrickson SC, St Louis JD, Lowe JE, Abdel-aleem S (1997) Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol. Cell. Biochem. 166: 85-94. DOI:10.1023/a:1006886601825.
58. An D, Rodrigues B (2006) Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 291: H1489-H1506. DOI:10.1152/ajpheart.00278.2006.
59. Heather LC, Clarke K (2011) Metabolism, hypoxia and the diabetic heart. J. Mol. Cell Cardiol. 50: 598–605.
60. Young ME, Guthrie PH, Razeghi P, Leighton B, Abbasi S, Patil S,Youker KA, Taegtmeyer H (2002) Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes 51: 2587–2595.
61. Gulick T, Cresci S, Caira T, Moore DD, Kelly DP (1994) The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc. Natl. Acad. Sci. 91: 11012–11016.
62. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, BaetensD, Orci L, and Unger RH (2000) Lipotoxic heart disease in obese rats:implications for human obesity. Proc. Natl. Acad. Sci. 97: 1784–1789.
63. Aasum E, Hafstad AD, Severson DL, and Larsen TS (2003) Age-dependentchanges in metabolism, contractile function, and ischemic sensitivity inhearts from db/db mice. Diabetes 52: 434–441.
64. Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW,Yun UJ, Cooksey RC, Litwin SE, and Abel ED (2005) Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 146: 5341–5349.
65. Chong CR, Clarke K, Levelt E (2017) Metabolic remodeling in diabetic cardiomyopathy. Cardiovasc. Res. 113: 422-430. DOI:10.1093/cvr/cvx018.
66. Entman ML, Bornet EP, Van Winkle WB, Goldstein MA, andSchwartz A (1977) Association of glycogenolysis with cardiac sarcoplasmicreticulum: I. Effect of glycogen depletion, deoxycholate solubilizationand cardiac ischemia: evidence for a phorphorylase kinase membranecomplex. J. Mol. Cell. Cardiol. 9: 515–528.
67. Lebeche D, Davidoff AJ, Hajjar RJ (2008) Interplay between impaired calciumregulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat. Clin. Pract. Cardiovasc. Med. 5: 715–724.
68. Zhou H, Yue Y, Wang J, Ma Q, Chen Y (2018) Melatonin therapy for diabetic cardiomyopathy: a mechanism involving Syk-mitochondrial complex I-SERCA pathway. Cell. Signal 47: 88-100. DOI: 10.1016/j.cellsig.2018.03.012.
69. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M (2016) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 37: 2129–2200.
70. Nabben M, Luiken JJFP, Glatz JFC (2018) Metabolic remodelling in heart failure revisited. Nat. Rev. Cardiol. 15: 780. DOI:10.1038/s41569-018-0115-8.
71. Karwi QG, Uddin GM, Ho KL, Lopaschuk GD (2018) Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 5: 68. DOI:10.3389/fcvm.2018.00068.
72. Wende AR, Brahma MK, McGinnis GR, Young ME (2017) Metabolic origins of heart failure. JACC Basic Transl. Sci. 2: 297-310. DOI:10.1016/j.jacbts.2016.11.009.
73. Glatz, J. F. C. & Luiken, J. J. F. P (2018) Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 59: 1084–1093.
74. Serviddio G, Bellanti F, Romano AD, Tamborra R, Rollo T, Altomare E, Vendemiale G (2007) Bioenergetics in aging: mitochondrial proton leak in aging rat liver, kidney and heart. Redox. Rep. 12: 91-95. DOI: 10.1179/135100007X162112.
75. Verma SK, Garikipati VNS, Kishore R (2017) Mitochondrial dysfunction and its impact on diabetic heart. Biochim. Biophys. Acta Mol. Basis Dis. 1863: 1098-1105. DOI: 10.1016/j.bbadis.2016.08.021.
76. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin— a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93: 350-84. DOI: 10.1016/j.pneurobio.2010.12.004.
77. Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: evolutionary history in animals and plants. Front. Endocrinol. 10: 249. DOI: 10.3389/fendo.2019.00249.
78. Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L (2010) Beneficial effects of melatonin in cardiovascular disease. Ann. Med. 42: 276-285. DOI: 10.3109/07853890903485748.
79. de Oliveira AC, Andreotti S, Farias Tda S, Torres-Leal FL, de Proença AR, Campaña AB, de Souza AH, Sertié RA, Carpinelli AR, Cipolla-Neto J, Lima FB (2012) Metabolic disorders and adipose tissue insulin responsiveness in neonatally STZ-induced diabetic rats are improved by longterm melatonin treatment. Endocrinology 153: 2178–2188. DOI: 10.1210/en.2011-1675.
80. Li Y, Li S, Zhou Y, Meng X, Zhang JJ, Xu DP, Li HB (2017) Melatonin for the prevention and treatment of cancer. Oncotarget 8: 39896-39921. DOI: 10.18632/oncotarget.16379.
81. Reiter RJ (1991) Melatonin: the chemical expression of darkness. Mol. Cell. Endocrinol. 79: C153-C158. DOI:10.1016/0303-7207(91)90087-9.
82. Dawson D, Armstrong SM (1996) Chronobiotics―drugs that shift rhythms. Pharmacol. Ther. 69: 15-36. DOI:10.1016/0163-7258(95)02020-9.
83. Laposky AD, Bass J, Kohsaka A, Turek FW (2008) Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 582: 142-151. DOI:10.1016/j.febslet.2007.06.079.
84. Zanquetta MM, Seraphim PM, Sumida DH, Cipolla-Neto J, Machado UF (2003) Calorie restriction reduces pinealectomy-induced insulin resistance by improving GLUT4 gene expression and its translocation to the plasma membrane. J. Pineal Res. 35: 141-148. DOI:10.1034/j.1600-079x.2003.00067.x.
85. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014) Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56: 371-381. DOI:10.1111/jpi.12137.
86. Acuña-Castroviejo D, Martín M, Macías M, Escames G, León J, Khaldy H, Reiter RJ (2001) Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 30: 65-74. DOI:10.1034/j.1600-079x.2001.300201.x.
87. Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W (2016) ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016: 4350965. DOI: https://doi.org/10.1155/2016/4350965.
88. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra MC, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181-197. DOI: 10.2174/1568026023394443.
89. Sarkar S, Chattopadhyay A, Bandyopadhyay D (2020) Melatonin, the advance-guard in oxidative myocardial assault instigated by exercise stress: a physiological and biochemical insight. Melatonin Res. 3: 451-475. DOI:https://doi.org/https://doi.org/10.32794/mr11250072.
90. Itani N, Skeffington KL, Beck C, Niu Y, Giussani DA (2016) Melatonin rescues cardiovascular dysfunction during hypoxic development in the chick embryo. J. Pineal Res. 60: 16-26. DOI: 10.1111/jpi.12283.
91. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66.
92. Prado NJ, Ferder L, Manucha W, Diez ER (2018) Anti-inflammatory effects of melatonin in obesity and hypertension. Curr. Hypertens. Rep. 20: 45. DOI: 10.1007/s11906-018-0842-6.
93. Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D (2000) Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 14: 1677-1679. DOI:10.1096/fj.99-0865fje.
94. Jiki Z, Lecour S, Nduhirabandi F (2018) Cardiovascular benefits of dietary melatonin: a myth or a reality?. Front. Physiol. 9: 528. DOI: 10.3389/fphys.2018.00528.
95. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González‐Gallego J (2013) A review of the molecular aspects of melatonin’s anti‐inflammatory actions: recent insights and new perspectives. J. Pineal Res. 54: 1-4. DOI:10.1111/j.1600-079X.2012.01014.x.
96. Nabavi SM, Nabavi SF, Sureda A, Xiao J, Dehpour AR, Shirooie S, Silva AS, Baldi A, Khan H, Daglia M (2019) Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 59: S4-S16. DOI: 10.1080/10408398.2018.1487927.
97. Sarkar S, Chattopadhyay A, Bandyopadhyay D (2021) Multiple strategies of melatonin protecting against cardiovascular injury related to inflammation: a comprehensive overview. Melatonin Res. 4: 1-29. DOI:https://doi.org/https://doi.org/10.32794/mr11250080.
98. Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Hölzenbein T, Markovic O, Leibetseder VJ, Strauss‐Blasche G, Marktl W (2003) The melatonin receptor subtype MT2 is present in the human cardiovascular system. J. Pineal Res. 35: 40-44. DOI: 10.1034/j.1600-079x.2003.00051.x.
99. Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ (2012) Melatonin and cardiovascular disease: myth or reality?. Rev. Esp. Cardiol. (English Edition) 65: 215-218. DOI: 10.1016/j.recesp.2011.10.009.
100. Zhao T, Zhang H, Jin C, Qiu F, Wu Y, Shi L (2017) Melatonin mediates vasodilation through both direct and indirect activation of BKCa channels. J Mol Endocrinol. 59: 219-233. DOI: 10.1530/JME-17-0028.
101. Reiter RJ, Tan DX & Korkmaz A (2009) The circadian melatonin rhythm and its modulation: possible impact on hypertension. J. Hypertens. 27: S17–S20. DOI:10.1097/01.hjh.0000358832.41181.bf.
102. Tunstall RR, Shukla P, Grazul-Bilska A, Sun C, O'Rourke ST (2011) MT2 receptors mediate the inhibitory effects of melatonin on nitric oxide-induced relaxation of porcine isolated coronary arteries. J. Pharmacol. Exp. Ther. 336: 127-133. DOI:10.1124/jpet.110.174482.
103. Escames G, López LC, Ortiz F, López A, García JA, Ros E, Acuña-Castroviejo D (2007) Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 274: 2135-2147. DOI:10.1111/j.1742-4658.2007.05755.x.
104. Reiter RJ, Tan DX, Kim SJ, Qi W (1998) Melatonin as a pharmacological agent against oxidative damage to lipids and DNA. Proc. West Pharmacol. Soc. 41: 229–236.
105. Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, Cao Z, Cheng Y, Wang BN, Wang Y (2013) Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR 4/NF‐κB system in high‐fat‐fed rabbits. J. Pineal Res. 55: 388-398. DOI: 10.1111/jpi.12085.
106. Lagneux C, Joyeux M, Demenge P, Ribuot C, Godin-Ribuot D (2000) Protective effects of melatonin against ischemia-reperfusion injury in the isolated rat heart. Life Sci. 66: 503-509. DOI: 10.1016/s0024-3205(99)00620-7.
107. Sahna E, Olmez E, Acet A (2002) Effects of physiological and pharmacological concentrations of melatonin on ischemia–reperfusion arrhythmias in rats: can the incidence of sudden cardiac death be reduced?. J. Pineal Res. 32: 194-198. DOI: 10.1034/j.1600-079x.2002.1o853.x.
108. Fu Z, Jiao Y, Wang J, Zhang Y, Shen M, Reiter RJ, Xi Q, Chen Y (2020) Cardioprotective Role of melatonin in acute myocardial infarction. Front. Physiol. 11: 366. DOI: 10.3389/fphys.2020.00366. PMID: 32411013; PMCID: PMC7201093.
109. Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA (2014) Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update. 20: 293-307. DOI: 10.1093/humupd/dmt054.
110. Valenzuela FJ, Vera J, Venegas C, Pino F, Lagunas C (2015) Circadian system and melatonin hormone: risk factors for complications during pregnancy. Obstet. Gynecol. Int. 2015: 825802. DOI: 10.1155/2015/825802.
111. Soliman A, Lacasse AA, Lanoix D, Sagrillo-Fagundes L, Boulard V, Vaillancourt C (2015) Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 59: 38-46. DOI: 10.1111/jpi.12236.
112. Ghosh G, De K, Maity S, Bandyopadhyay D, Bhattacharya S, Reiter RJ, Bandyopadhyay A (2007) Melatonin protects against oxidative damage and restores expression of GLUT4 gene in the hyperthyroid rat heart. J. Pineal Res. 42: 71-82.
113. Rosano GM, Vitale C (2018) Metabolic modulation of cardiac metabolism in heart failure. Card. Fail. Rev. 4: 99-103. DOI:10.15420/cfr.2018.18.2.
114. Oz E, Erbaş D, Sürücü HS, Düzgün E (2006) Prevention of doxorubicin-induced cardiotoxicity by melatonin. Mol. Cell. Biochem. 282: 31-37. DOI:10.1007/s11010-006-1153-9.
115. Mukherjee D, Roy SG, Bandyopadhyay A, Chattopadhyay A, Basu A, Mitra E, Ghosh AK, Reiter RJ, Bandyopadhyay D (2010) Melatonin protects against isoproterenol‐induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res. 48: 251-262. DOI: 10.1111/j.1600-079X.2010.00749.x.
116. Reiter RJ, Tan DX, Manchester LC, Terron MP, Flores LJ, Koppisepi S (2007) Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv. Med. Sci. 52: 11-28.
117. Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: a never‐ending interaction of melatonin with reactive oxygen and nitrogen species?. J. Pineal Res. 42: 28-42. DOI: 10.1111/j.1600-079X.2006.00407.x.
118. Sallam N, Laher I (2016) Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid. Med. Cell. Longev. 2016: 7239639. DOI: https://doi.org/10.1155/2016/7239639.
119. Lochner A, Marais E, Huisamen B (2018) Melatonin and cardioprotection against ischaemia/reperfusion injury: what's new? a review. J. Pineal Res. 65: e12490. DOI:10.1111/jpi.12490.
120. Taegtmeyer H, Sen S, Vela D (2010) Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann. NY Acad. Sci. 1188: 191–198. DOI: 10.1111/j.1749-6632.2009.05100.x.
121. Tomec RJ, Hoppel CL (1975) Carnitine palmitoyltransferase in bovine fetal heart mitochondria. Arch. Biochem. Biophys. 170: 716-723. DOI:10.1016/0003-9861(75)90169-1.
122. Hoppel CL, Kerner J, Turkaly P, Turkaly J, Tandler B (1998) The malonyl-CoA-sensitive form of carnitine palmitoyltransferase is not localized exclusively in the outer membrane of rat liver mitochondria. J. Biol. Chem. 273: 23495-23503. DOI:10.1074/jbc.273.36.23495.
123. Lopaschuk GD, Folmes CD, Stanley WC (2007) Cardiac energy metabolism in obesity. Circ. Res. 101: 335-347. DOI:10.1161/CIRCRESAHA.107.150417.
124. Song S, Attia RR, Connaughton S, Niesen MI, Ness GC, Elam MB, Hori RT, Cook GA, Park EA (2010) Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol. Cell. Endocrinol. 325: 54-63. DOI:10.1016/j.mce.2010.05.019.
125. Timm KN, Tyler DJ (2020) The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc. Drugs Ther. 34: 255-269. DOI:10.1007/s10557-020-06941-x.
126. Mi Y, Tan D, He Y, Zhou X, Zhou Q, Ji S (2018) Melatonin modulates lipid metabolism in HepG2 cells cultured in high concentrations of oleic acid: AMPK pathway activation may play an important role. Cell. Biochem. Biophys. 76: 463-470. DOI:10.1007/s12013-018-0859-0.
127. Wu S, Zou MH (2020) AMPK, mitochondrial function, and cardiovascular disease. Int. J. Mol. Sci. 21: 4987. DOI:10.3390/ijms21144987.
128. Shao D, Tian R (2015) Glucose Transporters in Cardiac Metabolism and Hypertrophy. Compr. Physiol. 6: 331-351. DOI:10.1002/cphy.c150016.
129. Chen Z, Liu M, Li L, Chen L (2018) Involvement of the Warburg effect in non-tumor diseases processes. J. Cell. Physiol. 233: 2839-2849. DOI:10.1002/jcp.25998.
130. Reiter RJ, Sharma R, Ma Q (2020) Switching diseased cells from cytosolic aerobic glycolysis to mitochondrial oxidative phosphorylation: A metabolic rhythm regulated by melatonin?. J. Pineal Res. 70: e12677. DOI:10.1111/jpi.12677.
131. Reiter RJ, Sharma R, Ma Q, Rosales-Corral S, Acuna-Castroviejo D, Escames G (2019) Inhibition of mitochondrial pyruvate dehydrogenase kinase: a proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2: 105-119. DOI: https://doi.org/10.32794/mr11250033.
132. Yeung HM, Hung MW, Lau CF, Fung ML (2015) Cardioprotective effects of melatonin against myocardial injuries induced by chronic intermittent hypoxia in rats. J. Pineal Res. 58: 12-25. DOI: 10.1111/jpi.12190.
133. van der Velden J, Tocchetti CG, Varricchi G, Bianco A, Sequeira V, Hilfiker-Kleiner D, Hamdani N, Leite-Moreira AF, Mayr M, Falcão-Pires I, Thum T, Dawson DK, Balligand JL, Heymans S (2018) Metabolic changes in hypertrophic cardiomyopathies: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology. Cardiovasc. Res. 114: 1273-1280. DOI:10.1093/cvr/cvy147.
134. Sinésio-Jr J, Bargi-Souza P, Matos RA, Leite EA, Buonfiglio DC, Andrade-Silva J, Motta-Teixeira L, Curi R, Young M, Cipolla-Neto J, Peliciari-Garcia RA (2019) Melatonin and the heart circadian clock of euglycemic and type 2 diabetic male rats: a transcriptional evaluation. Melatonin Res. 2: 139-151. DOI: https://doi.org/https://doi.org/10.32794/11250035.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


