Melatonin and biological membrane bilayers: a never ending amity
Melatonin protects biological membrane
Abstract
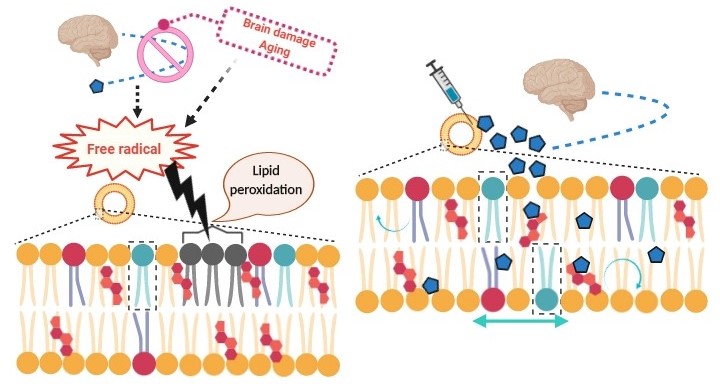
Biological membrane, the most fluidic structure of a cell or an organelle, refrains the cells to progress toward apoptosis by sustaining their optimum environment. This bilayer-membrane equips all machineries required for cellular communication, limits the entry of foreign bodies, selectively transports molecules or ions depending on the need of the system but, it also acts as a first line defense against environmental insults. Due to the presence of a vast number of poly unsaturated fatty acids (PUFA), the biological membrane is highly prone to oxidative stress and as a consequence, acceleration in lipid peroxidation by free radicals, becomes a threat to cellular viability. Alterations in the biophysical state of bilayer caused by oxidative stress frequently occur in the in vivo as well as in vitro conditions. It has been well documented that the molecule, melatonin, exhibits profound coherence in neutralizing oxidative stress and thus, to normalize fluidity status of biological membranes. Aging associated decline in melatonin level with subsequent ascended lipid peroxidation and membrane viscosity found in almost all organisms further suggest the importance of melatonin in this context. Since disruption of membrane structure or even some modifications will cause a spectrum of diseases, keeping membrane intactness would be an adequate strategy to prevent these diseases. Considering the high permeability, safe and potent antioxidant capacity of melatonin, this molecule can be a superlative choice to alleviate membrane bilayer rigidity and its related ailments.
References
2. Randow F, MacMicking JD, James LC (2013) Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science 340 (6133): 701-706. DOI: 10.1126/science.1233028.
3. Gaschler MM, Stockwell BR (2017) Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482 (3): 419-425. DOI: 10.1016/j.bbrc.2016.10.086.
4. Catalá A, Díaz M (2016) Impact of lipid peroxidation on the physiology and pathophysiology of cell membranes. Front. Physiol. 7: 423. DOI: 10.3389/fphys.2016.00423.
5. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre‐Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61 (3): 253-278. DOI: 10.1111/jpi.12360.
6. Karasek M (2004) Melatonin, human aging, and age-related diseases. Exp. Gerontol. 39 (11-12): 1723-9. DOI: 10.1016/j.exger.2004.04.012.
7. Manda K, Bhatia AL (2003) Melatonin-induced reduction in age-related accumulation of oxidative damage in mice. Biogerontology 4 (3): 133-9. DOI: 10.1023/a:1024102007763.
8. Alvarez E, Ruiz‐Gutiérrez V, Sobrino F, Santa-María C (2001) Age‐related changes in membrane lipid composition, fluidity and respiratory burst in rat peritoneal neutrophils. Clin. Exp. Immunol. 124 (1): 95-102. DOI: 10.1046/j.1365-2249.2001.01490.x.
9. Watson H (2015) Biological membranes. Essays Biochem. 59: 43-69. DOI: 10.1042/bse0590043.
10. Gulik-Krzywicki T (1975) Structural studies of the associations between biological membrane components. Biochim. Biophys. Acta 415 (1): 1-28. DOI: 10.1016/0304-4157(75)90015-5.
11. Bohdanowicz M, Grinstein S (2013) Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 93 (1): 69-106. DOI: 10.1152/physrev.00002.2012.
12. Manno S, Takakuwa Y, Mohandas N (2002) Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. 99 (4): 1943-1948. DOI: 10.1073/pnas.042688399.
13. Alenghat FJ, Golan DE (2013) Membrane protein dynamics and functional implications in mammalian cells. In: Current topics in membranes Vol. 72, pp. 89-120. Academic Press.
14. Marčelja S (1976) Lipid-mediated protein interaction in membranes. Biochim. Biophys. Acta 455 (1): 1-7. DOI: 10.1016/0005-2736(76)90149-8.
15. Lee AG (2003) Lipid–protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612 (1): 1-40. DOI: 10.1016/s0005-2736(03)00056-7.
16. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell 4th ed. New York: Garland Science.
17. García JJ, López-Pingarrón L, Almeida-Souza P, Tres A, Escudero P, García‐Gil FA, Tan DX, Reiter RJ, Ramírez JM, Bernal-Pérez M (2014) Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J. Pineal Res. 56 (3): 225-237. DOI: 10.1111/jpi.12128.
18. Girotti AW (1998) Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 39 (8): 1529-1542. PMID: 9717713.
19. Garcı́a JJ, Reiter RJ, Guerrero JM, Escames G, Byung PY, Oh CS, Muñoz-Hoyos A (1997) Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 408 (3): 297-300. DOI: 10.1016/s0014-5793(97)00447-x.
20. Garcia JJ, Reiter RJ, Ortiz GG, Oh CS, Tang L, Yu BP, Escames G (1998) Melatonin enhances tamoxifen's ability to prevent the reduction in microsomal membrane fluidity induced by lipid peroxidation. J. Membr. Biol. 162 (1): 59-65. DOI: 10.1007/s002329900342.
21. Reiter RJ, Tan DX, Galano A (2014) Melatonin reduces lipid peroxidation and membrane viscosity. Front. Physiol. 5: 377. DOI: 10.3389/fphys.2014.00377.
22. García JJ, Piñol-Ripoll G, Martínez-Ballarín E, Fuentes-Broto L, Miana-Mena FJ, Venegas C, Caballero B, Escames G, Coto-Montes A, Acuña-Castroviejo D (2011) Melatonin reduces membrane rigidity and oxidative damage in the brain of SAMP8 mice. Neurobiol. Aging 32 (11): 2045-2054. DOI: 10.1016/j.neurobiolaging.2009.12.013.
23. Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014: 360438. DOI: 10.1155/2014/360438.
24. Halliwell B, Gutteridge JM (1985) Free radicals in biology and medicine. pp 22-85. Clarendon Press, Oxford.
25. Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, USA.
26. Potapenko A, Roschupkin D, Kogan E, Vladimirov Yu (1972) Dokladi Academii Nauk SSSR 202, 882.
27. Dobretsov GE, Borschevskaya TA, Petrov VA, Vladimirov YA (1977) The increase of phospholipid bilayer rigidity after lipid peroxidation. FEBS Lett. 84 (1): 125-128. DOI: 10.1016/0014-5793(77)81071-5.
28. Schroeder F (1984) Role of membrane lipid asymmetry in aging. Neurobiol. Aging 5 (4): 323-333. DOI: 10.1016/0197-4580(84)90010-1.
29. Chen JJ, Yu BP (1994) Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic. Biol. Med. 17 (5): 411-418. DOI: 10.1016/0891-5849(94)90167-8.
30. Curtis MT, Gilfor D, Farber JL (1984) Lipid peroxidation increases the molecular order of microsomal membranes. Arch. Biochem. Biophys. 235 (2): 644-649. DOI: 10.1016/0003-9861(84)90239-x.
31. Singer S (1974) The molecular organization of membranes. Annu. Rev. Biochem. 43 (1): 805-833. DOI: 10.1146/annurev.bi.43.070174.004105.
32. Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L (2007) Effect of lipid peroxidation on the properties of lipid bilayer: a molecular dynamics study. Biophys. J. 93 (12): 4225-4236. DOI: 10.1529/biophysj.107.112565.
33. Schneider C, Boeglin WE, Yin H, Porter NA, Brash AR (2008) Intermolecular peroxyl radical reactions during autoxidation of hydroxy and hydroperoxy arachidonic acids generate a novel series of epoxidized products. Chem. Res. Toxicol. 21 (4): 895-903. DOI: 10.1021/tx700357u.
34. McGrath LT, Douglas AF, McClean E, Brown JH, Doherty CC, Johnston GD, Archbold GP (1995) Oxidative stress and erythrocyte membrane fluidity in patients undergoing regular dialysis. Clin. Chim. Acta 235 (2): 179-188. DOI: 10.1016/0009-8981(95)06027-x.
35. Choe M, Jackson C, Yu BP (1995) Lipid peroxidation contributes to age-related membrane rigidity. Free Radic. Biol. Med. 18 (6): 977-984. DOI: 10.1016/0891-5849(94)00217-8.
36. Goldberg DM, Riordan JR (1986) Role of membranes in disease. Clin. Physiol. Biochem. 4 (5): 305-336. PMID: 3022980.
37. Ademowo OS, Dias HK, Burton DG, Griffiths HR (2017) Lipid (per) oxidation in mitochondria: an emerging target in the ageing process? Biogerontology 18 (6): 859-879. DOI: 10.1007/s10522-017-9710-z.
38. Volinsky R, Kinnunen PK (2013) Oxidized phosphatidylcholines in membrane‐level cellular signaling: from biophysics to physiology and molecular pathology. FEBS J. 280 (12): 2806-2816. DOI: 10.1111/febs.12247.
39. Kinnunen PK, Kaarniranta K, Mahalka AK (2012) Protein-oxidized phospholipid interactions in cellular signaling for cell death: From biophysics to clinical correlations. Biochim. Biophys. Acta 1818 (10): 2446-2455. DOI: 10.1016/j.bbamem.2012.04.008.
40. Pizzimenti S, Ciamporcero ES, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, Minelli R, Dianzani C, Lepore A, Gentile F, Barrera G (2013) Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 4: 242. DOI: 10.3389/fphys.2013.00242.
41. Ma DW, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS (2004) n− 3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J. Nutr. Biochem. 15 (11): 700-706. DOI: 10.1016/j.jnutbio.2004.08.002.
42. Naudí A, Cabré R, Dominguez-Gonzalez M, Ayala V, Jové M, Mota-Martorell N, Piñol-Ripoll G, Gil-Villar MP, Rué M, Portero-Otín M, Ferrer I (2017) Region-specific vulnerability to lipid peroxidation and evidence of neuronal mechanisms for polyunsaturated fatty acid biosynthesis in the healthy adult human central nervous system. Biochim. Biophys. Acta 1862 (5): 485-495. DOI: 10.1016/j.bbalip.2017.02.001.
43. Dias HI, Brown CL, Polidori MC, Lip GY, Griffiths HR (2015) LDL-lipids from patients with hypercholesterolaemia and Alzheimer's disease are inflammatory to microvascular endothelial cells: mitigation by statin intervention. Clin. Sci. 129 (12): 1195-1206. DOI: 10.1042/CS20150351.
44. Lee R, Margaritis M, M Channon K, Antoniades C (2012) Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr. Med. Chem. 19 (16): 2504-2520. DOI: 10.2174/092986712800493057.
45. Anderson EJ, Katunga LA, Willis MS (2012) Mitochondria as a source and target of lipid peroxidation products in healthy and diseased heart. Clin. Exp. Pharmacol. Physiol. 39 (2): 179-193. DOI: 10.1111/j.1440-1681.2011.05641.x.
46. Nwose EU, Jelinek HF, Richards RS, Kerr PG (2007) Erythrocyte oxidative stress in clinical management of diabetes and its cardiovascular complications. Br. J. Biomed. Sci. 64 (1): 35-43. DOI: 10.1080/09674845.2007.11732754.
47. Morita M, Ishida N, Uchiyama K, Yamaguchi K, Itoh Y, Shichiri M, Yoshida Y, Hagihara Y, Naito Y, Yoshikawa T, Niki E (2012) Fatty liver induced by free radicals and lipid peroxidation. Free Radic. Res. 46 (6): 758-765. DOI: 10.3109/10715762.2012.677840.
48. Vaziri ND, Norris K (2011) Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purif. 31 (1-3): 189-196. DOI: 10.1159/000321845.
49. Sarban S, Kocyigit A, Yazar M, Isikan UE (2005) Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin. Biochem. 38 (11): 981-986. DOI: 10.1016/j.clinbiochem.2005.08.003.
50. Jaganjac M, Tirosh O, Cohen G, Sasson S, Zarkovic N (2013) Reactive aldehydes–second messengers of free radicals in diabetes mellitus. Free Radic. Res. 47 (sup1): 39-48. DOI: 10.3109/10715762.2013.789136.
51. Kennedy MA, Moffat TC, Gable K, Ganesan S, Niewola-Staszkowska K, Johnston A, Nislow C, Giaever G, Harris LJ, Loewith R, Zaremberg V (2016) A signaling lipid associated with Alzheimer’s disease promotes mitochondrial dysfunction. Sci. Rep. 6: 19332. DOI: 10.1038/srep19332.
52. Hong JH, Kang JW, Kim DK, Baik SH, Kim KH, Shanta SR, Jung JH, Mook-Jung I, Kim KP (2016) Global changes of phospholipids identified by MALDI imaging mass spectrometry in a mouse model of Alzheimer’s disease. J. Lipid Res. 57 (1): 36-45. DOI: 10.1194/jlr.M057869.
53. Usatyuk PV, Natarajan V (2012) Hydroxyalkenals and oxidized phospholipids modulation of endothelial cytoskeleton, focal adhesion and adherens junction proteins in regulating endothelial barrier function. Microvasc. Res. 83 (1): 45-55. DOI: 10.1016/j.mvr.2011.04.012.
54. Fritz KS, Petersen DR (2011) Exploring the biology of lipid peroxidation-derived protein carbonylation. Chem. Res. Toxicol. 24 (9): 1411-1419. DOI: 10.1021/tx200169n.
55. Jain SK, McVie R, Duett J, Herbst JJ (1989) Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 38 (12): 1539-1543. 10.2337/diab.38.12.1539.
56. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin. Interv. Aging 13: 757. DOI: 10.2147/CIA.S158513.
57. Butterfield DA, Lange ML, Sultana R (2010) Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim. Biophys. Acta 1801 (8): 924-929. DOI: 10.1016/j.bbalip.2010.02.005.
58. Wallace DC (1992) Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science 256 (5057): 628-632. DOI: 10.1126/science.1533953.
59. Mecocci P, Cherubini A, Beal MF, Cecchetti R, Chionne F, Polidori MC, Romano G, Senin U (1996) Altered mitochondrial membrane fluidity in AD brain. Neurosci. Lett. 207 (2): 129-132. DOI: 10.1016/0304-3940(96)12509-x.
60. Hajimohammadreza I, Brammer M (1990) Brain membrane fluidity and lipid peroxidation in Alzheimer's disease. Neurosci. Lett. 112 (2-3): 333-337. DOI: 10.1016/0304-3940(90)90226-y.
61. Chia LS, Thompson JE, Moscarello MA (1984) X-ray diffraction evidence for myelin disorder in brain from humans with Alzheimer's disease. Biochim. Biophys. Acta 775 (3): 308-312. DOI: 10.1016/0005-2736(84)90185-8.
62. Reddy PH (2009) Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp. Neurol. 218 (2): 286-292. DOI: 10.1016/j.expneurol.2009.03.042.
63. Sultana R, Butterfield DA (2009) Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J. Bioenerg. Biomembr. 41 (5): 441-446. DOI: 10.1007/s10863-009-9241-7.
64. Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR (1998) Regional membrane phospholipid alterations in Alzheimer's disease. Neurochem. Res. 23 (1): 81-88. DOI: 10.1023/a:1022457605436.
65. Markesbery WR, Lovell MA (1998) Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging 19 (1): 33-36. DOI: 10.1016/s0197-4580(98)00009-8.
66. Kotha SR, Gurney TO, Magalang MU, Hund TJ, Satoskar AR, Mohler PJ, Maddipati KR, Natarajan V, Parinandi NL (2014) Mitochondrial lipid peroxidation in lung damage and disease. In: Mitochondrial function in lung health and disease pp. 117-139. Humana Press, New York, NY.
67. Pytel E, Jackowska P, Chwatko G, Olszewska-Banaszczyk M, Koter-Michalak M, Kubalczyk P, Broncel M (2016) Intensive statin therapy, used alone or in combination with ezetimibe, improves homocysteine level and lipid peroxidation to a similar degree in patients with coronary artery diseases. Pharmacol. Rep. 68 (2): 344-348. DOI: 10.1016/j.pharep.2015.08.019.
68. Ceraulo L, Ferrugia M, Tesoriere L, Segreto S, Livrea MA, Liveri VT (1999) Interactions of melatonin with membrane models: portioning of melatonin in AOT and lecithin reversed micelles. J. Pineal Res. 26 (2): 108-112. DOI: 10.1111/j.1600-079x.1999.tb00570.x.
69. Costa EJ, Lopes RH, Lamy-Freund MT (1995) Permeability of pure lipid bilayer to melatonin. J. Pineal Res. 19 (3): 123-126. DOI: 10.1111/j.1600-079x.1995.tb00180.x.
70. Tan DX, Pöeggeler B, Reiter RJ, Chen LD, Chen S, Lucien MC, Barlow-Walden LR (1993) The pineal hormone melatonin inhibits DNA-adduct formation induced by the chemical carcinogen safrole in vivo. Cancer Lett. 70 (1-2): 65-71. DOI: 10.1016/0304-3835(93)90076-l.
71. Reiter RJ, Poeggeler B, Tan DX, Chen LD, Manchester LC (1993) Antioxidant capacity of melatonin: a novel action not requiring a receptor. Neuroendocrinol. Lett. 15 (1-2):103-116.
72. Shida CS, Castrucci AD, Lamy‐Freund MT (1994) High melatonin solubility in aqueous medium. J. Pineal Res. 16 (4): 198-201. DOI: 10.1111/j.1600-079x.1994.tb00102.x.
73. Cardinali DP, Lynch HJ, Wurtman RJ (1972) Binding of melatonin to human and rat plasma proteins. Endocrinol. 91 (5): 1213-1218. DOI: 10.1210/endo-91-5-1213.
74. Melchiorri D, Reiter RJ, Attia AM, Hara M, Burgos A, Nistico G (1994) Potent protective effect of melatonin on in vivo paraquat-induced oxidative damage in rats. Life Sci. 56 (2): 83-89. DOI: 10.1016/0024-3205(94)00417-q.
75. Costa EJ, Shida CS, Biaggi MH, Ito AS, Lamy-Freund MT (1997) How melatonin interacts with lipid bilayer: a study by fluorescence and ESR spectroscopies. FEBS Lett. 416 (1): 103-106. DOI: 10.1016/s0014-5793(97)01178-2.
76. Acufla-Castroviejo D, Escames G, Macias M, Hoyos AM, Carballo AM, Arauzo M, Montes R, Vives F (1995) Minireview: cell protective role of melatonin in the brain. J. Pineal Res. 19 (2): 57-63. DOI: 10.1111/j.1600-079X.1995.tb00171.x.
77. Garcia JJ, Reiter RJ, Pie J, Ortiz GG, Cabrera J, Sainz RM, Acuña-Castroviejo D (1999) Role of pinoline and melatonin in stabilizing hepatic microsomal membranes against oxidative stress. J. Bioenerg. Biomembr. 31 (6): 609-616. DOI: 10.1023/a:1005425213253.
78. Fischer T, Bangha E, Elsner P, Kistler GS (1999) Suppression of UV-induced Erythema by topical treatment with melatonin. Influence of the application time point. Biol. Signals Recept. 8 (1-2): 132-135. DOI: 10.1159/000014581.
79. Saija A, Tomaino A, Trombetta D, Pellegrino ML, Tita B, Caruso S, Castelli F (2002) Interaction of melatonin with model membranes and possible implications in its photoprotective activity. Eur. J. Pharm. Biopharm. 53 (2): 209-215. DOI: 10.1016/s0939-6411(01)00239-9.
80. Tesoriere L, D'arpa D, Conti S, Giaccone V, Pintaudi AM, Livrea MA (1999) Melatonin protects human red blood cells from oxidative hemolysis: New insights into the radical scavenging activity. J. Pineal Res. 27 (2): 95-105. DOI: 10.1111/j.1600-079x.1999.tb00602.x.
81. Bongiorno D, Ceraulo L, Ferrugia M, Filizzola F, Giordano C, Ruggirello A, Liveri VT (2004) H-NMR and FT-IR study of the state of melatonin confined in membrane models: location and interactions of melatonin in water free lecithin and AOT reversed micelles. Arkivoc. 251: 262.
82. Bongiorno D, Ceraulo L, Ferrugia M, Filizzola F, Ruggirello A, Liveri VT (2005) Localization and interactions of melatonin in dry cholesterol/lecithin mixed reversed micelles used as cell membrane models. J. Pineal Res. 38 (4): 292-298. DOI: 10.1111/j.1600-079X.2005.00211.x.
83. de Lima VR, Caro MS, Tavares MI, Creczynski-Pasa TB (2007) Melatonin location in egg phosphatidylcholine liposomes: possible relation to its antioxidant mechanisms. J. Pineal Res. 43 (3): 276-282. DOI: 10.1111/j.1600-079X.2007.00474.x.
84. de Lima VR, Morfim MP, Teixeira A, Creczynski-Pasa TB (2004) Relationship between the action of reactive oxygen and nitrogen species on bilayer membranes and antioxidants. Chem. Phys. Lipids 132 (2): 197-208. DOI: 10.1016/j.chemphyslip.2004.07.003.
85. Chen TY, Lee MY, Chen HY, Kuo YL, Lin SC, Wu TS, Lee EJ (2006) Melatonin attenuates the postischemic increase in blood–brain barrier permeability and decreases hemorrhagic transformation of tissue plasminogen activator therapy following ischemic stroke in mice. J. Pineal Res. 40 (3): 242-250. DOI: 10.1111/j.1600-079X.2005.00307.x.
86. Johns J (2011) Estimation of melatonin blood brain barrier permeability. J. Bioanal. Biomed. 3 (3): 64-69. DOI: 10.4172/1948-593X.
87. Dies H, Cheung B, Tang J, Rheinstädter MC (2015) The organization of melatonin in lipid membranes. Biochim. Biophys. Acta 1848 (4): 1032-1040. DOI: 10.1016/j.bbamem.2015.01.006.
88. Sahin I, Severcan F, Kazancı N (2007) Melatonin induces opposite effects on order and dynamics of anionic DPPG model membranes. J. Mol. Struc. 834: 195-201.DOI: 10.1016/j.molstruc.2006.12.002.
89. Dies H, Toppozini L, Rheinstädter MC (2014) The interaction between amyloid-β peptides and anionic lipid membranes containing cholesterol and melatonin. PLoS One 9 (6): e99124. DOI: 10.1371/journal.pone.0099124.
90. Choucair A, Chakrapani M, Chakravarthy B, Katsaras J, Johnston LJ (2007) Preferential accumulation of Aβ (1− 42) on gel phase domains of lipid bilayer: An AFM and fluorescence study. Biochim. Biophys. Acta 1768 (1): 146-154. DOI: 10.1016/j.bbamem.2006.09.005.
91. Lu H, Marti J (2020) Cellular absorption of small molecules: free energy landscapes of melatonin binding at phospholipid membranes. Sci. Rep. 10 (1): 1-2. DOI: 10.1038/s41598-020-65753-z.
92. Lu H, Martí J (2019) Binding and dynamics of melatonin at the interface of phosphatidylcholine-cholesterol membranes. PloS ONE 14 (11): e0224624. DOI: 10.1371/journal.pone.0224624.
93. Yu H, Dickson EJ, Jung SR, Koh DS, Hille B (2016) High membrane permeability for melatonin. J. Gen. Physiol. 147 (1): 63-76. DOI: 10.1085/jgp.201511526.
94. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B (1996) Evaluation of the antioxidant activity of melatonin in vitro. Free Radic. Biol. Med. 21 (3): 307-315. DOI: 10.1016/0891-5849(96)00046-9
95. Marchetti C, Sidahmed-Adrar N, Collin F, Jore D, Gardès-Albert M, Bonnefont-Rousselot D (2011) Melatonin protects PLPC liposomes and LDL towards radical-induced oxidation. J. Pineal Res. 51 (3): 286-296. DOI: 10.1111/j.1600-079X.2011.00889.x.
96. Mekhloufi J, Vitrac H, Yous S, Duriez P, Jore D, Gardès-Albert M, Bonnefont-Rousselot D (2007) Quantification of the water/lipid affinity of melatonin and a pinoline derivative in lipid models. J. Pineal Res. 42 (4): 330-337. DOI: 10.1111/j.1600-079X.2007.00423.x.
97. Livrea MA, Tesoriere L, D’Arpa D, Morreale M (1997) Reaction of melatonin with lipoperoxyl radicals in phospholipid bilayer. Free Radic. Biol. Med. 23 (5): 706-711. DOI: 10.1016/s0891-5849(97)00018-x.
98. Yamamoto HA, Tang HW (1996) Melatonin attenuates L-cysteine induced seizures and lipid peroxidation in the brain of mice. J. Pineal Res. 21 (2): 108-113. DOI: 10.1111/j.1600-079x.1996.tb00277.x.
99. Li XJ, Zhang LM, Gu J, Zhang AZ, Sun FY (1997) Melatonin decreases production of hydroxyl radical during cerebral ischemia-reperfusion. Acta Pharmacologica Sinica. 18 (5): 394-396. PMID: 10322924.
100. Carneiro RC, Reiter RJ (1997) Melatonin protects against lipid peroxidation induced by δ-aminolevulinic acid in rat cerebellum, cortex and hippocampus. Neuroscience 82 (1): 293-299. DOI: 10.1016/s0306-4522(97)00262-5.
101. Melchiorri D, Reiter RJ, Sewerynek E, Chen LD, Nistic G (1995) Melatonin reduces kainite induced lipid peroxidation in homogenates of different brain regions. FASEB J. 9 (12): 1205-1210. DOI: 10.1096/fasebj.9.12.7672513.
102. Princ FG, Juknat AA, Maxit AG, Cardalda C, Battle A (1997) Melatonin's antioxidant protection against δ-aminolevulinic acid induced oxidative damage in rat cerebellum. J. Pineal Res. 23 (1): 40-46. DOI: 10.1111/j.1600-079x.1997.tb00333.x.
103. Reiter R, Tang L, Garcia JJ, Muñoz-Hoyos A (1997) Pharmacological actions of melatonin in oxygen radical pathophysiology. Life sci. 60 (25): 2255-2271. DOI: 10.1016/s0024-3205(97)00030-1.
104. Leaden P, Barrionuevo J, Catalá A (2002) The protection of long chain polyunsaturated fatty acids by melatonin during nonenzymatic lipid peroxidation of rat liver microsomes. J. Pineal Res. 32 (3): 129-134. DOI: 10.1034/j.1600-079x.2002.1o829.x.
105. Teixeira A, Morfim MP, de Cordova CA, Charão CC, de Lima VR, Creczynski‐Pasa TB (2003) Melatonin protects against pro-oxidant enzymes and reduces lipid peroxidation in distinct membranes induced by the hydroxyl and ascorbyl radicals and by peroxynitrite. J. Pineal Res. 35 (4): 262-268. DOI: 10.1034/j.1600-079x.2003.00085.x.
106. Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1: 57-60.
107. Matuszak Z, Reszka KJ, Chignell CF (1997) Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic. Biol. Med. 23 (3): 367-372. DOI: 10.1016/s0891-5849(96)00614-4.
108. Stasica P, Ulanski P, Rosiak JM (1998) Melatonin as a hydroxyl radical scavenger. J. Pineal Res. 25: 65-66. DOI: 10.1111/j.1600-079x.1998.tb00387.x.
109. Cuzzocrea S, Zingarelli B, Gilad E, Hake P, Salzman AL, Szabó C (1997) Protective effect of melatonin in carrageenan induced models of local inflammation: relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. J. Pineal Res. 23 (2): 106-116. DOI: 10.1111/j.1600-079x.1997.tb00342.x.
110. Zavodnik IB, Domanski AV, Lapshina EA, Bryszewska M, Reiter RJ (2006) Melatonin directly scavenges free radicals generated in red blood cells and a cell-free system: chemiluminescence measurements and theoretical calculations. Life Sci. 79 (4): 391-400. DOI: 10.1016/j.lfs.2006.01.030.
111. Arnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress?. Trends Plant Sci. 19 (12): 789-797. DOI: 10.1016/j.tplants.2014.07.006.
112. Karbownik M, Reiter RJ, Garcia JJ, Tan DX (2000) Melatonin reduces phenylhydrazine-induced oxidative damage to cellular membranes: evidence for the involvement of iron. Int. J. Biochem. Cell Biol. 32 (10):1045-1054. DOI: 10.1016/s1357-2725(00)00056-x.
113. Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. 54 (3): 245-257. DOI: 10.1111/jpi.12010.
114. Galano A, Tan DX, Reiter RJ (2014) Cyclic 3-hydroxymelatonin, a key metabolite enhancing the peroxyl radical scavenging activity of melatonin. RSC Adv. 4 (10): 5220-5227. DOI: 10.1039/C3RA44604B.
115. Poeggeler B, Saarela S, Reiter RJ, TAN DX, CHEN LD, Manchester LC, Barlow-walden LR (1994) Melatonin—a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 738 (1): 419-420. DOI: 10.1111/j.1749-6632.1994.tb21831.x.
116. Tan DX, Hardeland R, Manchester LC, Galano A, Reiter RJ (2014) Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Curr. Med. Chem. 21 (13): 1557-1565. DOI: 10.2174/0929867321666131129113146.
117. Schaefer M, Hardeland R (2009) The melatonin metabolite N1‐acetyl‐5‐methoxykynuramine is a potent singlet oxygen scavenger. J. Pineal Res. 46 (1): 49-52. DOI: 10.1111/j.1600-079X.2008.00614.x.
118. Galano A (2011) On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 13 (15): 7178-7188. DOI: 10.1039/c0cp02801k.
119. Xia MZ, Liang YL, Wang H, Chen X, Huang YY, Zhang ZH, Chen YH, Zhang C, Zhao M, Xu DX, Song LH (2012) Melatonin modulates TLR4-mediated inflammatory genes through MyD88 and TRIF dependent signaling pathways in lipopolysaccharide‐stimulated RAW264.7 cells. J. Pineal Res. 53 (4): 325-334. DOI: 10.1111/j.1600-079X.2012.01002.x.
120. León J, Escames G, Rodríguez MI, López LC, Tapias V, Entrena A, Camacho E, Carrión MD, Gallo MA, Espinosa A, Tan DX (2006) Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 98 (6): 2023-2033. DOI: 10.1111/j.1471-4159.2006.04029.x.
121. Schaffazick SR, Pohlmann AR, De Cordova CA, Creczynski-Pasa TB, Guterres SS (2005) Protective properties of melatonin-loaded nanoparticles against lipid peroxidation. Int. J. Pharm. 289 (1-2): 209-213. DOI: 10.1016/j.ijpharm.2004.11.003.
122. Karbownik M, Reiter RJ, Qi W, Garcia JJ, Tan DX, Manchester LC (2000) Protective effects of melatonin against oxidation of guanine bases in DNA and decreased microsomal membrane fluidity in rat liver induced by whole body ionizing radiation. Mol. Cell Biochem. 211 (1-2): 137-144. DOI: 10.1023/a:1007148530845.
123. Ferber E, Kröner E, Schmidt B, Fischer H, Peskar BA, Anders C (1980) Dynamics of membrane fatty acids during lymphocyte stimulation by mitogens. In: Membrane Fluidity pp. 239-263. Humana Press.
124. Ostro MJ, Bessinger B, Summers J, Dray S (1980) Effect of membrane lipid composition on mobility of lymphocyte surface immunoglobulins. In: Membrane Fluidity pp. 105-117. Humana Press.
125. Hegner D (1980) Age-dependence of molecular and functional changes in biological membrane properties. Mech. Ageing Dev. 14 (1-2): 101-118. DOI: 10.1016/0047-6374(80)90109-8.
126. Shinitzky M (1984) Membrane fluidity in malignancy adversative and recuperative. Biochim. Biophys. Acta 738 (4): 251-261. DOI: 10.1016/0304-419x(83)90007-0.
127. Tsuda K, Nishio I (2003) Membrane fluidity and hypertension. Am. J. Hypertens 16: 259-261. DOI: 10.1016/s0895-7061(02)03257-0.
128. Zimmer G, Thurich T, Scheer B, Packer L, Fuchs J (1993) Membrane fluidity and vitamin E In: Packer L, Fuchs J (eds) Vitamin E in health and disease pp- 207-222 New York: Marcel Dekker.
129. Dzikovski B., Freed J. (2013) Membrane fluidity. In: Roberts G.C.K. (eds) Encyclopedia of Biophysics. Springer, Berlin, Heidelberg. DOI: 10.1007/978-3-642-16712-6_546.
130. Cooper RA (1977) Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N. Engl. J. Med. 297 (7): 371-377. DOI: 10.1056/NEJM197708182970707.
131. van Blitterswijk WJ (1988) Structural basis and physiological control of membrane fluidity in normal and tumor cells. In: Fluorescence Studies on Biological Membranes pp. 393-413. Springer, Boston, MA.
132. Lakatta EG, Zhou YY, Xiao RP, Boluyt M (2001) Aging of the Cardiovascular System. In: Heart Physiology and Pathophysiology (Fourth Edition) pp. 737-760. Academic Press. DOI: 10.1016/B978-012656975-9/50044-4.
133. García-Gil FA, Albendea CD, López-Pingarrón L, Royo-Dachary P, Martínez-Guillén J, Piedrafita E, Martínez-Díez M, Soria J, García JJ (2012) Altered cellular membrane fluidity levels and lipid peroxidation during experimental pancreas transplantation. J. Bioenerg. Biomembr. 44 (5): 571-577. DOI: 10.1007/s10863-012-9459-7.
134. Hitzemann RJ, Hirschowitz J, Garver DL (1986) On the physical properties of red cell ghost membranes in the affective disorders and psychoses: a fluorescence polarization study. J. Affect. Disord. 10 (3): 227-232. DOI: 10.1016/0165-0327(86)90009-1.
135. Miana-Mena FJ, Piedrafita E, González-Mingot C, Larrodé P, Muñoz MJ, Martínez-Ballarín E, Reiter RJ, Osta R, García JJ (2011) Levels of membrane fluidity in the spinal cord and the brain in an animal model of amyotrophic lateral sclerosis. J. Bioenerg. Biomembr. 43 (2): 181. DOI: 10.1007/s10863-011-9348-5.
136. Los DA, Murata N (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 1666 (1-2): 142-157. DOI: 10.1016/j.bbamem.2004.08.002.
137. Curtis MT, Gilfor D, Farber JL (1984) Lipid peroxidation increases the molecular order of microsomal membranes. Arch. Biochem Biophysics. 235 (2): 644-649. DOI: 10.1016/0003-9861(84)90239-x.
138. Yu BP, Suescun EA, Yang SY (1992) Effect of age-related lipid peroxidation on membrane fluidity and phospholipase A2: modulation by dietary restriction. Mech. Ageing Dev. 65 (1): 17-33. DOI: 10.1016/0047-6374(92)90123-u.
139. Ghosh C, Dick RM, Ali SF (1993) Iron/ascorbate-induced lipid peroxidation changes membrane fluidity and muscarinic cholinergic receptor binding in rat frontal cortex. Neurochem. Int. 23 (5): 479-484. DOI: 10.1016/0197-0186(93)90133-p.
140. Kaplán P, Račay P, Lehotský J, Mézešová V (1995) Change in fluidity of brain endoplasmic reticulum membranes by oxygen free radicals: A protective effect of stobadine, α-tocopherol acetate, and butylated hydroxytoluene. Neurochem. Res. 20 (7): 815-820. DOI: 10.1007/BF00969693.
141. Eichenberger K, Böhni P, Winterhalter KH, Kawato S, Richter C (1982) Microsomal lipid peroxidation causes an increase in the order of the membrane lipid domain. FEBS Lett. 142 (1): 59-62. DOI: 10.1016/0014-5793(82)80219-6.
142. Rice-Evans C, Hochstein P (1981) Alterations in erythrocyte membrane fluidity by phenylhydrazine-induced peroxidation of lipids. Biochem. Biophys. Res. Commun. 100 (4): 1537-1542. DOI: 10.1016/0006-291x(81)90693-8.
143. Watanabe H, Kobayashi A, Yamamoto T, Suzuki S, Hayashi H, Yamazaki N (1990) Alterations of human erythrocyte membrane fluidity by oxygen-derived free radicals and calcium. Free Radic. Biol. Med. 8 (6): 507-514. DOI: 10.1016/0891-5849(90)90150-h.
144. Rudzite V, Jurika E, Jirgensons J (1999) Changes in membrane fluidity induced by tryptophan and its metabolites. In: Tryptophan, Serotonin, and Melatonin pp. 353-367. Springer, Boston, MA.
145. McLean LR, Hagaman KA (1992) Effect of lipid physical state on the rate of peroxidation of liposomes. Free Radic. Biol. Med. 12 (2): 113-119. DOI: 10.1016/0891-5849(92)90004-z.
146. Daniels WM, Van Rensburg SJ, Van Zyl JM, Van Der Walt BJ, Taljaard JJ (1996) Free radical scavenging effects of melatonin and serotonin: possible mechanism. Neuroreport 7 (10): 1593-1596. DOI: 10.1097/00001756-199607080-00012.
147. Ochoa JJ, Vílchez MJ, Palacios MA, García JJ, Reiter RJ, Muñoz‐Hoyos A (2003) Melatonin protects against lipid peroxidation and membrane rigidity in erythrocytes from patients undergoing cardiopulmonary bypass surgery. J. Pineal Res. 35 (2): 104-108. DOI: 10.1034/j.1600-079x.2003.00061.x.
148. Severcan F, Sahin I, Kazancı N (2005) Melatonin strongly interacts with zwitterionic model membranes—evidence from Fourier transform infrared spectroscopy and differential scanning calorimetry. Biochim. Biophys. Acta 1668 (2): 215-22. DOI: 10.1016/j.bbamem.2004.12.009. DOI: 10.1016/j.bbamem.2004.12.009.
149. Karbownik M, Reiter RJ, Garcia JJ, Tan DX, Qi W, Manchester LC (2000) Melatonin reduces rat hepatic macromolecular damage due to oxidative stress caused by δ-aminolevulinic acid. Biochim. Biophys. Acta 1523 (2-3): 140-146. DOI: 10.1016/s0304-4165(00)00110-0.
150. Aranda M, Albendea CD, Lostale F, López‐Pingarrón L, Fuentes‐Broto L, Martínez‐Ballarín E, Reiter RJ, Pérez‐Castejón MC, Garcia JJ (2010) In vivo hepatic oxidative stress because of carbon tetrachloride toxicity: protection by melatonin and pinoline. J. Pineal Res. 49 (1):78-85. DOI: 10.1111/j.1600-079X.2010.00769.x.
151. Calvo JR, Reiter RJ, Garcia JJ, Ortiz GG, Tan DX, Karbownik M (2001) Characterization of the protective effects of melatonin and related indoles against α‐naphthylisothiocyanate‐induced liver injury in rats. J. Cell Biochem. 80 (4): 461-470. DOI: 10.1002/1097-4644(20010315)80:4<461::aid-jcb1000>3.0.co;2-p.
152. Reiter RJ, Richardson BA, Johnson LY, Ferguson BN, Dinh DT (1980) Pineal melatonin rhythm: reduction in aging Syrian hamsters. Science 210 (4476): 1372-1373. DOI: 10.1126/science.7434032.
153. Reiter RJ, Craft CM, Johnson JR JE, King TS, Richardson BA, Vaughan GM, Vaughan MK (1981) Age-associated reduction in nocturnal pineal melatonin levels in female rats. Endocrinology 109 (4): 1295-1297. DOI: 10.1210/endo-109-4-1295.
154. Reiter RJ, Tan DX, Kim SJ, Manchester LC, Qi W, Garcia JJ, Cabrera JC, El-Sokkary G, Rouvier-Garay V (1999) Augmentation of indices of oxidative damage in life-long melatonin-deficient rats. Mech. Ageing Dev. 110 (3):157-173. DOI: 10.1016/s0047-6374(99)00058-5.
155. Ohvo-Rekilä H, Ramstedt B, Leppimäki P, Slotte JP (2002) Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41 (1): 66-97. DOI: 10.1016/s0163-7827(01)00020-0.
156. Bonn M, Roke S, Berg O, Juurlink LB, Stamouli A, Müller M (2004) A molecular view of cholesterol-induced condensation in a lipid monolayer. J. Phys. Chem. B. 108 (50): 19083-19085. DOI: 10.1021/jp0452249.
157. Moroni F, Marcheselli F, Recchioni R, Fattoretti P, Bertoni-Freddari C (2004) Pineal graft in old rats improves erythrocyte resistance to peroxyl radical-induced hemolysis. Biogerontology 5 (5): 339-344. DOI: 10.1007/s10522-004-2572-1.
158. Rodríguez MI, Escames G, López LC, López A, García JA, Ortiz F, Sánchez V, Romeu M, Acuña-Castroviejo D (2008) Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp. Gerontol. 43 (8): 749-756. DOI: 10.1016/j.exger.2008.04.003.
159. Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 17 (12): 2124. DOI: 10.3390/ijms17122124.
160. Hevia D, Gonzalez-Menendez P, Quiros-Gonzalez I, Miar A, Rodriguez-Garcia A, Tan DX, Reiter RJ, Mayo JC, Sainz RM (2015) Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 58: 234–250. DOI: 10.1111/jpi.12210.
161. Reiter RJ, Fuentes-Broto L, Paredes SD, Tan DX, Garcia JJ (2010) Melatonin and the pathophysiology of cellular membranes. Marmara Pharm. J. 14: 1-9. DOI: 10.12991/201014457.
162. Mayo JC, Cernuda R, Quiros I, Rodriguez P, Garcia JI, Hevia D, Sainz RM (2019) Understanding the role of melatonin in cancer metabolism. Melatonin Res. 2 (3): 76-104. DOI: 10.32794/11250032.
163. Hevia D, Sainz RM, Blanco D, Quirós I, Tan DX, Rodríguez C, Mayo JC (2008) Melatonin uptake in prostate cancer cells: intracellular transport versus simple passive diffusion. J. Pineal Res. 45 (3): 247-257. DOI: 10.1111/j.1600-079X.2008.00581.x.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


